The Envelope Cytoplasmic Tail of HIV-1 Subtype C Contributes to Poor Replication Capacity through Low Viral Infectivity and Cell-to-Cell Transmission
- PMID: 27598717
- PMCID: PMC5012655
- DOI: 10.1371/journal.pone.0161596
The Envelope Cytoplasmic Tail of HIV-1 Subtype C Contributes to Poor Replication Capacity through Low Viral Infectivity and Cell-to-Cell Transmission
Abstract
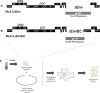
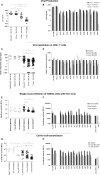
The cytoplasmic tail (gp41CT) of the HIV-1 envelope (Env) mediates Env incorporation into virions and regulates Env intracellular trafficking. Little is known about the functional impact of variability in this domain. To address this issue, we compared the replication of recombinant virus pairs carrying the full Env (Env viruses) or the Env ectodomain fused to the gp41CT of NL4.3 (EnvEC viruses) (12 subtype C and 10 subtype B pairs) in primary CD4+ T-cells and monocyte-derived-macrophages (MDMs). In CD4+ T-cells, replication was as follows: B-EnvEC = B-Env>C-EnvEC>C-Env, indicating that the gp41CT of subtype C contributes to the low replicative capacity of this subtype. In MDMs, in contrast, replication capacity was comparable for all viruses regardless of subtype and of gp41CT. In CD4+ T-cells, viral entry, viral release and viral gene expression were similar. However, infectivity of free virions and cell-to-cell transmission of C-Env viruses released by CD4+ T-cells was lower, suggestive of lower Env incorporation into virions. Subtype C matrix only minimally rescued viral replication and failed to restore infectivity of free viruses and cell-to-cell transmission. Taken together, these results show that polymorphisms in the gp41CT contribute to viral replication capacity and suggest that the number of Env spikes per virion may vary across subtypes. These findings should be taken into consideration in the design of vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Blanco J, Bosch B, Fernandez-Figueras MT, Barretina J, Clotet B, Este JA. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. The Journal of biological chemistry. 2004;279(49):51305–14. Epub 2004/09/17. 10.1074/jbc.M408547200 M408547200 [pii]. . - DOI - PubMed
-
- Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. Journal of virology. 2007;81(22):12582–95. Epub 2007/08/31. JVI.00381-07 [pii] 10.1128/JVI.00381-07 - DOI - PMC - PubMed
-
- Ruggiero E, Bona R, Muratori C, Federico M. Virological consequences of early events following cell-cell contact between human immunodeficiency virus type 1-infected and uninfected CD4+ cells. Journal of virology. 2008;82(16):7773–89. Epub 2008/05/30. JVI.00695-08 [pii] 10.1128/JVI.00695-08 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

