Modulation of infection-mediated migration of neutrophils and CXCR2 trafficking by osteopontin
- PMID: 27599164
- PMCID: PMC5341506
- DOI: 10.1111/imm.12668
Modulation of infection-mediated migration of neutrophils and CXCR2 trafficking by osteopontin
Abstract
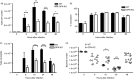
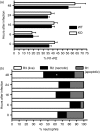
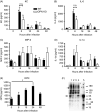
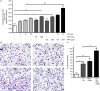
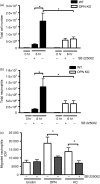
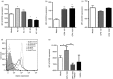
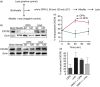
Osteopontin (OPN) is a pro-inflammatory protein that paradoxically protects against inflammation and bone destruction in a mouse model of endodontic infection. Here we have tested the hypothesis that this effect of OPN is mediated by effects on migration of innate immune cells to the site of infection. Using the air pouch as a model of endodontic infection in mice, we showed that neutrophil accumulation at the site of infection with a mixture of endodontic pathogens is significantly reduced in OPN-deficient mice. Reduced neutrophil accumulation in the absence of OPN was accompanied by an increase in bacterial load. OPN-deficiency did not affect neutrophil survival, CXCR2 ligand expression, or the production of inflammatory cytokines in the air pouch. In vitro, OPN enhanced neutrophil migration to CXCL1, whereas in vivo, inhibition of CXCR2 suppressed cellular infiltration in air pouches of infected wild-type mice by > 50%, but had no effect in OPN-deficient mice. OPN increased cell surface expression of CXCR2 on bone marrow neutrophils in an integrin-αv -dependent manner, and suppressed the internalization of CXCR2 in the absence of ligand. Together, these results support a model where the protective effect of OPN results from enhanced initial neutrophil accumulation at sites of infection resulting in optimal bacterial killing. We describe a novel mechanism for this effect of OPN: integrin-αv -dependent suppression of CXCR2 internalization in neutrophils, which increases the ability of these cells to migrate to sites of infection in response to CXCR2 ligands.
Keywords: CXCR2; endodontic infection; integrin-αv; osteopontin; receptor recycling.
© 2016 John Wiley & Sons Ltd.
Figures
Similar articles
-
Protective role of osteopontin in endodontic infection.Immunology. 2010 Jan;129(1):105-14. doi: 10.1111/j.1365-2567.2009.03159.x. Epub 2009 Sep 11. Immunology. 2010. PMID: 19824920 Free PMC article.
-
CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-β.Int J Cancer. 2014 Mar 15;134(6):1346-58. doi: 10.1002/ijc.28551. Epub 2013 Oct 31. Int J Cancer. 2014. PMID: 24154944
-
Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFα and CXCL1/CXCR2 in mice.Can J Physiol Pharmacol. 2012 Feb;90(2):187-99. doi: 10.1139/y11-116. Epub 2012 Feb 9. Can J Physiol Pharmacol. 2012. PMID: 22320712
-
Combined anti CXC receptors 1 and 2 therapy is a promising anti-inflammatory treatment for respiratory diseases by reducing neutrophil migration and activation.Pulm Pharmacol Ther. 2015 Oct;34:37-45. doi: 10.1016/j.pupt.2015.08.002. Epub 2015 Aug 10. Pulm Pharmacol Ther. 2015. PMID: 26271598 Review.
-
A key regulator of tumor-associated neutrophils: the CXCR2 chemokine receptor.J Mol Histol. 2024 Dec;55(6):1051-1061. doi: 10.1007/s10735-024-10260-y. Epub 2024 Sep 13. J Mol Histol. 2024. PMID: 39269537 Review.
Cited by
-
Effect of ORF7 of SARS-CoV-2 on the Chemotaxis of Monocytes and Neutrophils In Vitro.Dis Markers. 2021 Sep 28;2021:6803510. doi: 10.1155/2021/6803510. eCollection 2021. Dis Markers. 2021. PMID: 34603560 Free PMC article.
-
Baseline serum levels of osteopontin predict clinical response to treatment with nivolumab in patients with non-small cell lung cancer.Clin Exp Metastasis. 2019 Oct;36(5):449-456. doi: 10.1007/s10585-019-09984-z. Epub 2019 Aug 2. Clin Exp Metastasis. 2019. PMID: 31376097 Clinical Trial.
-
Chemokine (C-C Motif) Receptor-Like 2 is not essential for lung injury, lung inflammation, or airway hyperresponsiveness induced by acute exposure to ozone.Physiol Rep. 2017 Dec;5(24):e13545. doi: 10.14814/phy2.13545. Physiol Rep. 2017. PMID: 29242308 Free PMC article.
-
Immunoregulatory Roles of Osteopontin in Diseases.Nutrients. 2024 Jan 20;16(2):312. doi: 10.3390/nu16020312. Nutrients. 2024. PMID: 38276550 Free PMC article. Review.
-
Plasma osteopontin versus intima media thickness of the common carotid arteries in well-characterised patients with systemic lupus erythematosus.Lupus. 2021 Jul;30(8):1244-1253. doi: 10.1177/09612033211013898. Epub 2021 May 6. Lupus. 2021. PMID: 33957796 Free PMC article.
References
-
- Figdor D. Apical periodontitis: a very prevalent problem. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002; 94:651–2. - PubMed
-
- Siqueira JF Jr, Rocas IN. Diversity of endodontic microbiota revisited. J Dent Res 2009; 88:969–81. - PubMed
-
- Sasaki H, Hou L, Belani A, Wang CY, Uchiyama T, Muller R et al IL‐10, but not IL‐4, suppresses infection‐stimulated bone resorption in vivo . J Immunol 2000; 165:3626–30. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

