Inhibition of YAP suppresses CML cell proliferation and enhances efficacy of imatinib in vitro and in vivo
- PMID: 27599610
- PMCID: PMC5012077
- DOI: 10.1186/s13046-016-0414-z
Inhibition of YAP suppresses CML cell proliferation and enhances efficacy of imatinib in vitro and in vivo
Abstract
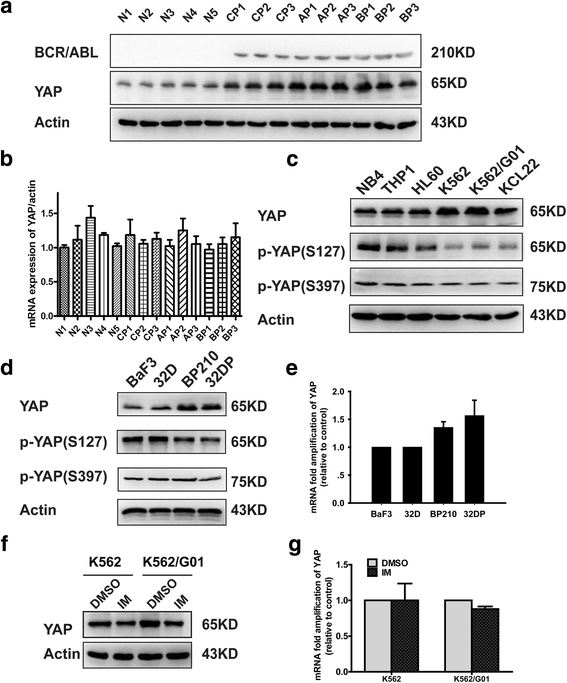
Background: Yes-associated protein (YAP), an essential component of Hippo pathway, was identified as an oncoprotein which participated in the progression of various malignancies. However, its role in chronic myeloid leukemia (CML) remains to be further clarified.
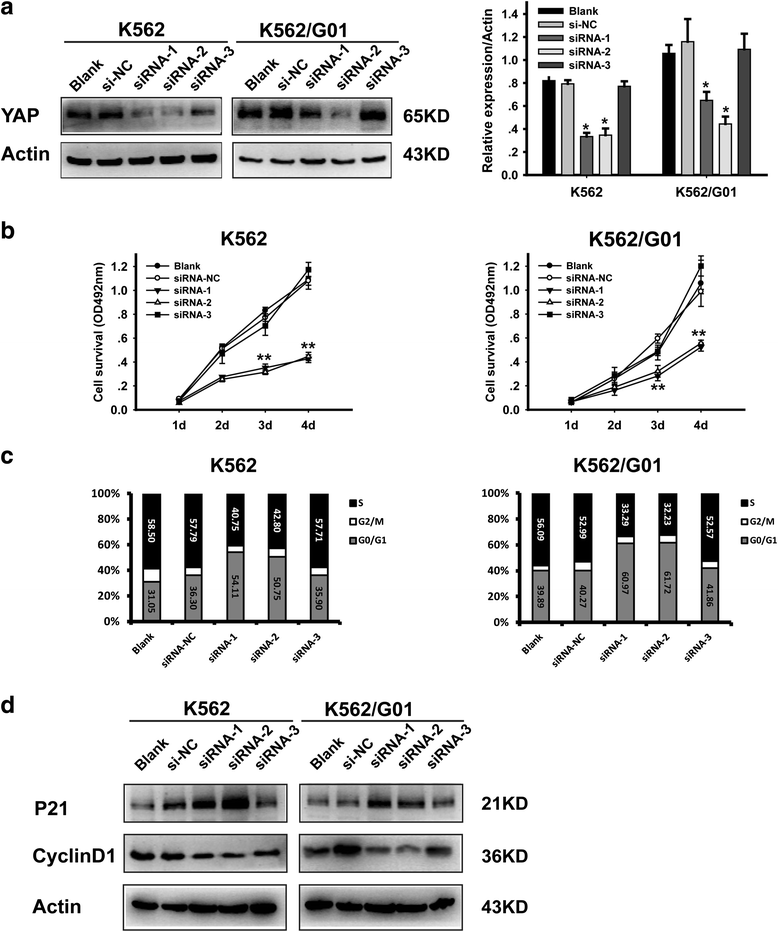
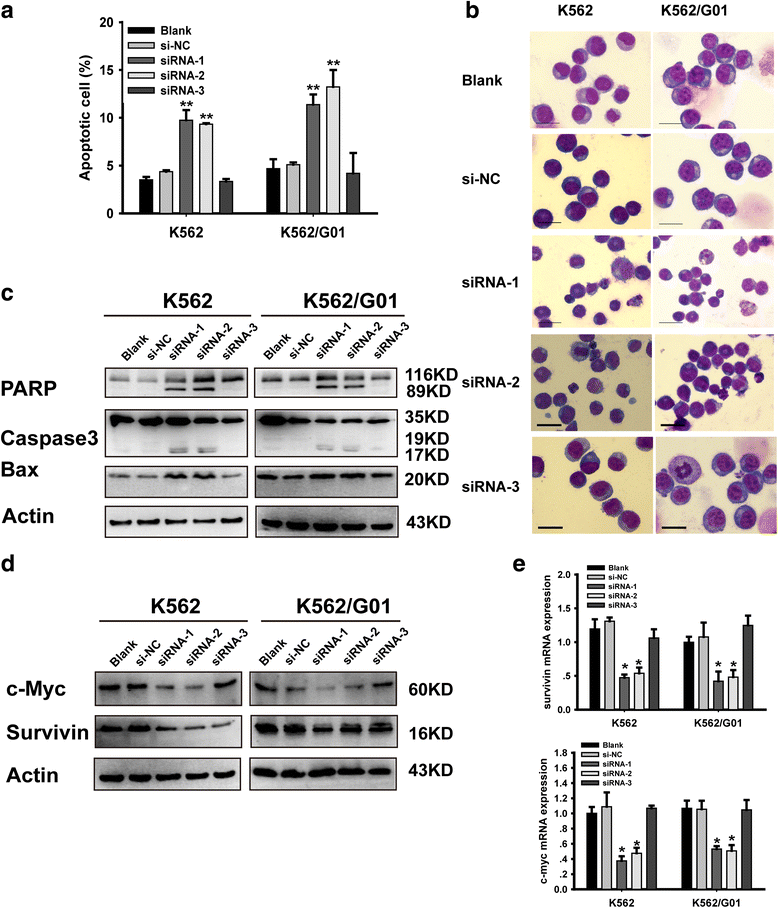
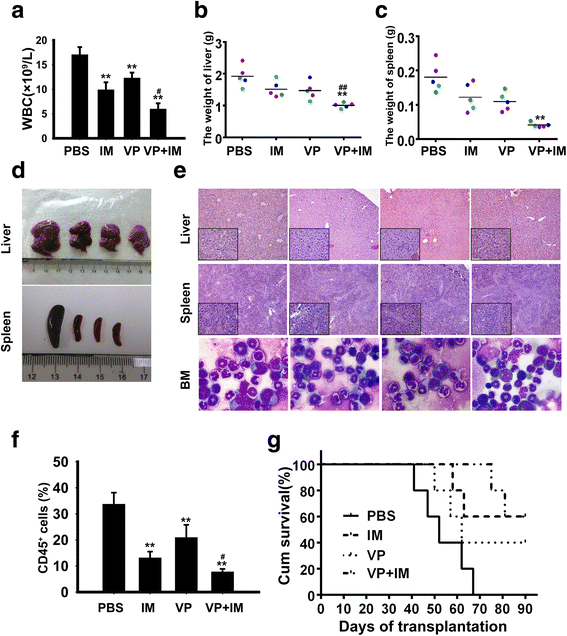
Methods: The expression of YAP in CML cells was determined by western blotting. Next, the effects of YAP knockdown and YAP inhibitor on CML cells were evaluated by MTT assay, flow cytometry (FCM) and Wright's staining. Moreover, K562 induced mice model was employed to further investigate the role of YAP in vivo.
Results: YAP was overexpressed in CML cells. Knockdown of YAP by si-RNA or inhibition the function of YAP using verteporfin (VP) not only inhibited the proliferation, induced the apoptosis of CML cells but also reduced the expression of YAP target genes c-myc and survivin. Additionally, VP enhanced the efficacy of imatinib (IM) in vitro and suppressed leukemogenesis in vivo.
Conclusion: Our results indicate that YAP may play an important role in the proliferation and leukemogenesis of CML cells. Genetic or pharmacological inhibition of YAP provides a novel treatment strategy for CML.
Keywords: Bcr/Abl; Chronic myeloid leukemia; IM; Leukemogenesis; Verteporfin; YAP.
Figures

Similar articles
-
Effect of YAP Inhibition on Human Leukemia HL-60 Cells.Int J Med Sci. 2017 Jul 20;14(9):902-910. doi: 10.7150/ijms.19965. eCollection 2017. Int J Med Sci. 2017. PMID: 28824329 Free PMC article.
-
Long-Term Exposure to Imatinib Mesylate Downregulates Hippo Pathway and Activates YAP in a Model of Chronic Myelogenous Leukemia.Stem Cells Dev. 2017 May 1;26(9):656-677. doi: 10.1089/scd.2016.0262. Epub 2017 Feb 27. Stem Cells Dev. 2017. PMID: 28103766 Free PMC article.
-
Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex.Cancer Sci. 2017 Mar;108(3):478-487. doi: 10.1111/cas.13138. Cancer Sci. 2017. PMID: 28002618 Free PMC article.
-
EPS8 regulates proliferation, apoptosis and chemosensitivity in BCR-ABL positive cells via the BCR-ABL/PI3K/AKT/mTOR pathway.Oncol Rep. 2018 Jan;39(1):119-128. doi: 10.3892/or.2017.6102. Epub 2017 Nov 20. Oncol Rep. 2018. PMID: 29192326 Free PMC article.
-
A brief review: some compounds targeting YAP against malignancies.Future Oncol. 2019 May;15(13):1535-1543. doi: 10.2217/fon-2019-0035. Epub 2019 May 8. Future Oncol. 2019. PMID: 31066301 Review.
Cited by
-
The tumor suppressor role of salvador family WW domain-containing protein 1 (SAV1): one of the key pieces of the tumor puzzle.J Cancer Res Clin Oncol. 2021 May;147(5):1287-1297. doi: 10.1007/s00432-021-03552-3. Epub 2021 Feb 12. J Cancer Res Clin Oncol. 2021. PMID: 33580421 Free PMC article. Review.
-
P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling.Circ Res. 2017 Nov 10;121(11):1224-1236. doi: 10.1161/CIRCRESAHA.117.310812. Epub 2017 Sep 18. Circ Res. 2017. PMID: 28923792 Free PMC article.
-
Boolean model of anchorage dependence and contact inhibition points to coordinated inhibition but semi-independent induction of proliferation and migration.Comput Struct Biotechnol J. 2020 Aug 4;18:2145-2165. doi: 10.1016/j.csbj.2020.07.016. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32913583 Free PMC article.
-
Disrupting myddosome assembly in diffuse large B‑cell lymphoma cells using the MYD88 dimerization inhibitor ST2825.Oncol Rep. 2019 Nov;42(5):1755-1766. doi: 10.3892/or.2019.7282. Epub 2019 Aug 19. Oncol Rep. 2019. PMID: 31432184 Free PMC article.
-
Automatic Multi-functional Integration Program (AMFIP) towards all-optical mechano-electrophysiology interrogation.PLoS One. 2022 Jul 28;17(7):e0266098. doi: 10.1371/journal.pone.0266098. eCollection 2022. PLoS One. 2022. PMID: 35901062 Free PMC article.
References
-
- Prieto F, Egozcue J, Forteza G, Marco F. Identification of the Philadelphia (Ph-1) chromosome. Blood. 1970;35:23–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

