Improved ligand geometries in crystallographic refinement using AFITT in PHENIX
- PMID: 27599738
- PMCID: PMC5013598
- DOI: 10.1107/S2059798316012225
Improved ligand geometries in crystallographic refinement using AFITT in PHENIX
Abstract
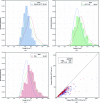
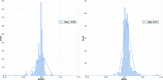
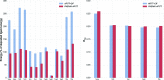
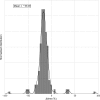
Modern crystal structure refinement programs rely on geometry restraints to overcome the challenge of a low data-to-parameter ratio. While the classical Engh and Huber restraints work well for standard amino-acid residues, the chemical complexity of small-molecule ligands presents a particular challenge. Most current approaches either limit ligand restraints to those that can be readily described in the Crystallographic Information File (CIF) format, thus sacrificing chemical flexibility and energetic accuracy, or they employ protocols that substantially lengthen the refinement time, potentially hindering rapid automated refinement workflows. PHENIX-AFITT refinement uses a full molecular-mechanics force field for user-selected small-molecule ligands during refinement, eliminating the potentially difficult problem of finding or generating high-quality geometry restraints. It is fully integrated with a standard refinement protocol and requires practically no additional steps from the user, making it ideal for high-throughput workflows. PHENIX-AFITT refinements also handle multiple ligands in a single model, alternate conformations and covalently bound ligands. Here, the results of combining AFITT and the PHENIX software suite on a data set of 189 protein-ligand PDB structures are presented. Refinements using PHENIX-AFITT significantly reduce ligand conformational energy and lead to improved geometries without detriment to the fit to the experimental data. For the data presented, PHENIX-AFITT refinements result in more chemically accurate models for small-molecule ligands.
Keywords: AFITT; PHENIX; geometry restraints; ligands; macromolecular refinement.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

