3D Cell Culture in Alginate Hydrogels
- PMID: 27600217
- PMCID: PMC4996398
- DOI: 10.3390/microarrays4020133
3D Cell Culture in Alginate Hydrogels
Abstract
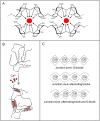
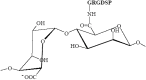
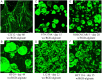
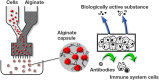
This review compiles information regarding the use of alginate, and in particular alginate hydrogels, in culturing cells in 3D. Knowledge of alginate chemical structure and functionality are shown to be important parameters in design of alginate-based matrices for cell culture. Gel elasticity as well as hydrogel stability can be impacted by the type of alginate used, its concentration, the choice of gelation technique (ionic or covalent), and divalent cation chosen as the gel inducing ion. The use of peptide-coupled alginate can control cell-matrix interactions. Gelation of alginate with concomitant immobilization of cells can take various forms. Droplets or beads have been utilized since the 1980s for immobilizing cells. Newer matrices such as macroporous scaffolds are now entering the 3D cell culture product market. Finally, delayed gelling, injectable, alginate systems show utility in the translation of in vitro cell culture to in vivo tissue engineering applications. Alginate has a history and a future in 3D cell culture. Historically, cells were encapsulated in alginate droplets cross-linked with calcium for the development of artificial organs. Now, several commercial products based on alginate are being used as 3D cell culture systems that also demonstrate the possibility of replacing or regenerating tissue.
Keywords: 3D; AlgiMatrix®; NovaMatrix®-3D; alginate; beads; bioprinting; drug development; drug discovery; hydrogel; tissue regeneration.
Figures



References
-
- Gevaert M. Engineering 3D Tissue Systems to Better Mimic Human Biology. Bridge. 2012;42:48–55.
-
- Kenny P.A., Lee G.Y., Myers C.A., Neve R.M., Semeiks J.R., Spellman P.T., Lorenz K., Lee E.H., Barcellos-Hoff M.H., Petersen O.W., et al. The Morphologies of Breast Cancer Cell Lines in Three-Dimensional Assays Correlate With Their Profiles of Gene Expression. Mol. Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. - DOI - PMC - PubMed
-
- Paul S.M., Mytelka D.S., Dunwiddie C.T., Persinger C.C., Munos B.H., Lindborg S.R., Schacht A.L. How to Improve R&D Productivity: The Pharmaceutical Industry's Grand Challenge. Nat. Rev. Drug Discov. 2010;9:203–214. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

