Synergistic effect of N-decorated and Mn(2+) doped ZnO nanofibers with enhanced photocatalytic activity
- PMID: 27600260
- PMCID: PMC5013319
- DOI: 10.1038/srep32711
Synergistic effect of N-decorated and Mn(2+) doped ZnO nanofibers with enhanced photocatalytic activity
Abstract
Here we report a high efficiency photocatalyst, i.e., Mn(2+)-doped and N-decorated ZnO nanofibers (NFs) enriched with vacancy defects, fabricated via electrospinning and a subsequent controlled annealing process. This nanocatalyst exhibits excellent visible-light photocatalytic activity and an apparent quantum efficiency up to 12.77%, which is 50 times higher than that of pure ZnO. It also demonstrates good stability and durability in repeated photocatalytic degradation experiments. A comprehensive structural analysis shows that high density of oxygen vacancies and nitrogen are introduced into the nanofibers surface. Hence, the significant enhanced visible photocatalytic properties for Mn-ZnO NFs are due to the synergetic effects of both Mn(2+) doping and N decorated. Further investigations exhibit that the Mn(2+)-doping facilitates the formation of N-decorated and surface defects when annealing in N2 atmosphere. N doping induce the huge band gap decrease and thus significantly enhance the absorption of ZnO nanofibers in the range of visible-light. Overall, this paper provides a new approach to fabricate visible-light nanocatalysts using both doping and annealing under anoxic ambient.
Figures


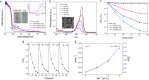


 0) surface. (b) Calculated DOS of ZnO and 4.2% Mn2+-doped ZnO in the form of a bulk crystal. (c,d) Electron densities of states of bulk ZnO and 4.2% Mn2+-doped ZnO. The energy of the valence band maximum of the bulk phase is taken to be zero.
0) surface. (b) Calculated DOS of ZnO and 4.2% Mn2+-doped ZnO in the form of a bulk crystal. (c,d) Electron densities of states of bulk ZnO and 4.2% Mn2+-doped ZnO. The energy of the valence band maximum of the bulk phase is taken to be zero.
Similar articles
-
Facile fabrication of Mn2+-doped ZnO photocatalysts by electrospinning.R Soc Open Sci. 2020 Apr 22;7(4):191050. doi: 10.1098/rsos.191050. eCollection 2020 Apr. R Soc Open Sci. 2020. PMID: 32431858 Free PMC article.
-
Designed Ag-decorated Mn:ZnO nanocomposite: facile synthesis, and enhanced visible light absorption and photogenerated carrier separation.Phys Chem Chem Phys. 2020 Dec 7;22(46):27272-27279. doi: 10.1039/d0cp04731g. Phys Chem Chem Phys. 2020. PMID: 33227105
-
Multi-shelled ZnO decorated with nitrogen and phosphorus co-doped carbon quantum dots: synthesis and enhanced photodegradation activity of methylene blue in aqueous solutions.RSC Adv. 2019 Mar 6;9(13):7362-7374. doi: 10.1039/c9ra00168a. eCollection 2019 Mar 1. RSC Adv. 2019. PMID: 35519954 Free PMC article.
-
Enhanced Visible Light Photocatalytic Activity of ZnO Nanowires Doped with Mn2+ and Co2+ Ions.Nanomaterials (Basel). 2017 Jan 19;7(1):20. doi: 10.3390/nano7010020. Nanomaterials (Basel). 2017. PMID: 28336854 Free PMC article.
-
Strategies to Enhance ZnO Photocatalyst's Performance for Water Treatment: A Comprehensive Review.Chem Rec. 2022 Jul;22(7):e202100299. doi: 10.1002/tcr.202100299. Epub 2022 Feb 4. Chem Rec. 2022. PMID: 35119182 Review.
Cited by
-
Effect of thickness and reaction media on properties of ZnO thin films by SILAR.Sci Rep. 2022 Jan 17;12(1):851. doi: 10.1038/s41598-022-04782-2. Sci Rep. 2022. PMID: 35039553 Free PMC article.
-
Electrospun Ceramic Nanofibers for Photocatalysis.Nanomaterials (Basel). 2021 Nov 27;11(12):3221. doi: 10.3390/nano11123221. Nanomaterials (Basel). 2021. PMID: 34947570 Free PMC article. Review.
-
Microwave-Assisted Synthesis of SnO2@ZnIn2S4 Composites for Highly Efficient Photocatalytic Hydrogen Evolution.Materials (Basel). 2024 May 15;17(10):2367. doi: 10.3390/ma17102367. Materials (Basel). 2024. PMID: 38793432 Free PMC article.
-
Tuning of the band gap and dielectric loss factor by Mn doping of Zn1-xMnxO nanoparticles.Sci Rep. 2023 May 27;13(1):8646. doi: 10.1038/s41598-023-35456-2. Sci Rep. 2023. PMID: 37244922 Free PMC article.
-
Strong Volta potential change in doped zinc oxide as a photoresponse to UV irradiation.RSC Adv. 2019 Nov 1;9(61):35579-35587. doi: 10.1039/c9ra01758e. eCollection 2019 Oct 31. RSC Adv. 2019. PMID: 35528075 Free PMC article.
References
-
- Elumalai N. K., Vijila C., Jose R., Uddin A. & Ramakrishna S. Metal oxide semiconducting interfacial layers for photovoltaic and photocatalytic applications. Mater Renew Sustain Energy 131–169 (2012).
-
- Xu S. & Wang Z. L. One-dimensional ZnO nanostructures: Solution growth and functional properties. Nano Research 4, 1013–1098 (2011).
-
- Zhou H. & Wong S. S. A Facile and Mild Synthesis of 1-D ZnO, CuO, and α-Fe2O3 Nanostructures and Nanostructured Arrays. ACS Nano 2, 944–958 (2008). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

