[18F]FDG PET/CT-based response assessment of stage IV non-small cell lung cancer treated with paclitaxel-carboplatin-bevacizumab with or without nitroglycerin patches
- PMID: 27600280
- PMCID: PMC5121177
- DOI: 10.1007/s00259-016-3498-y
[18F]FDG PET/CT-based response assessment of stage IV non-small cell lung cancer treated with paclitaxel-carboplatin-bevacizumab with or without nitroglycerin patches
Abstract
Purpose: Nitroglycerin (NTG) is a vasodilating drug, which increases tumor blood flow and consequently decreases hypoxia. Therefore, changes in [18F] fluorodeoxyglucose positron emission tomography ([18F]FDG PET) uptake pattern may occur. In this analysis, we investigated the feasibility of [18F]FDG PET for response assessment to paclitaxel-carboplatin-bevacizumab (PCB) treatment with and without NTG patches. And we compared the [18F]FDG PET response assessment to RECIST response assessment and survival.
Methods: A total of 223 stage IV non-small cell lung cancer (NSCLC) patients were included in a phase II study (NCT01171170) randomizing between PCB treatment with or without NTG patches. For 60 participating patients, a baseline and a second [18F]FDG PET/computed tomography (CT) scan, performed between day 22 and 24 after the start of treatment, were available. Tumor response was defined as a 30 % decrease in CT and PET parameters, and was compared to RECIST response at week 6. The predictive value of these assessments for progression free survival (PFS) and overall survival (OS) was assessed with and without NTG.
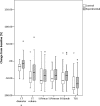
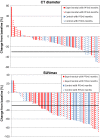
Results: A 30 % decrease in SUVpeak assessment identified more patients as responders compared to a 30 % decrease in CT diameter assessment (73 % vs. 18 %), however, this was not correlated to OS (SUVpeak30 p = 0.833; CTdiameter30 p = 0.557). Changes in PET parameters between the baseline and the second scan were not significantly different for the NTG group compared to the control group (p value range 0.159-0.634). The CT-based (part of the [18F]FDG PET/CT) parameters showed a significant difference between the baseline and the second scan for the NTG group compared to the control group (CT diameter decrease of 7 ± 23 % vs. 19 ± 14 %, p = 0.016, respectively).
Conclusions: The decrease in tumoral FDG uptake in advanced NSCLC patients treated with chemotherapy with and without NTG did not differ between both treatment arms. Early PET-based response assessment showed more tumor responders than CT-based response assessment (part of the [18F]FDG PET/CT); this was not correlated to survival. This might be due to timing of the [18F]FDG PET shortly after the bevacizumab infusion.
Keywords: Bevacizumab; Nitroglycerin; Response assessment; Stage IV NSCLC; [18F]FDG PET/CT.
Conflict of interest statement
Compliance with ethical standards Funding The NVALT12 is a multicentre randomized open-label parallel group phase II trial conducted by the Dutch Lung Physician Society (NVALT) and was supported by the Dutch Cancer Society (grant UM 2010–4883). This study is also funded by the Dutch Technology Foundation STW (grant n° 10696 DuCAT & n° P14-19 Radiomics STRaTegy), which is the applied science division of NWO and the Technology Programme of the Ministry of Economic Affairs. Conflict of interest Author RL is employed by ptTheragnostics BV and is the Chief Scientific Officer of OncoRadiomics (a department of Health Innovations B.V.). Author AD is an advisory board member of Roche, Eli Lilly, Astra Zeneca, MSD and Pfizer. Author PL acknowledges financial support from ERC advanced grant (ERC-ADG-2015, n° 694812 - Hypoximmuno), the QuIC-ConCePT project, which is partly funded by EFPI A companies and the Innovative Medicine Initiative Joint Undertaking (IMI JU) under grant agreement no. 115151. Author PL also acknowledges financial support from the EU 7th Framework Program (ARTFORCE - n° 257144), Kankeronderzoekfonds Limburg from the Health Foundation Limburg and the Dutch Cancer Society. Authors EJ, WE, OH, HG, RB, VN and ET declare that they have no conflicts of interest. Ethical approval All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments. Informed consent Informed consent was obtained from all individual participants included in the study.
Figures

References
-
- de Geus-Oei LF, van der Heijden HF, Visser EP, Hermsen R, van Hoorn BA, Timmer-Bonte JN, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J. Nucl. Med Off Pub Soc Nuc Med. 2007;48(10):1592–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

