Dabigatran antagonizes growth, cell-cycle progression, migration, and endothelial tube formation induced by thrombin in breast and glioblastoma cell lines
- PMID: 27600331
- PMCID: PMC5083743
- DOI: 10.1002/cam4.857
Dabigatran antagonizes growth, cell-cycle progression, migration, and endothelial tube formation induced by thrombin in breast and glioblastoma cell lines
Abstract
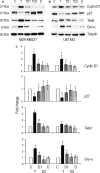
Thrombin activates its G-coupled seven transmembrane protease-activated receptor (PAR-1) by cleaving the receptor's N-terminal end. In several human cancers, PAR1 expression and activation correlates with tumor progression and metastatization. This provides compelling evidence for the effectiveness of an appropriate antithrombin agent for the adjuvant treatment of patients with cancer. Dabigatran is a selective direct thrombin inhibitor that reversibly binds to thrombin. In this study, we aimed to explore if dabigatran may affect mechanisms favoring tumor growth by interfering with thrombin-induced PAR-1 activation. We confirmed that exposure of tumor cells to thrombin significantly increased cell proliferation and this was coupled with downregulation of p27 and concomitant induction of cyclin D1. Dabigatran was consistently effective in antagonizing thrombin-induced proliferation as well as it restored the baseline pattern of cell cycle protein expression. Thrombin significantly upregulated the expression of proangiogenetic proteins like Twist and GRO-α in human umbilical vascular endothelial cells (HUVEC) cells and their expression was significantly brought down to control levels when dabigatran was added to culture. We also found that the chemoattractant effect of thrombin on tumor cells was lost in the presence of dabigatran, and that the thrombin antagonist was effective in dampening vascular tube formation induced by thrombin. Our data support a role of thrombin in inducing the proliferation, migration, and proangiogenetic effects of tumor cells in vitro. Dabigatran has activity in antagonizing all these effects, thereby impairing tumor growth and progression. In vivo models may help to understand the relevance of this pathway.
Keywords: Cancer; PAR-1; dabigatran; thrombin.
© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures





Similar articles
-
Thrombin induces tumor cell cycle activation and spontaneous growth by down-regulation of p27Kip1, in association with the up-regulation of Skp2 and MiR-222.Cancer Res. 2009 Apr 15;69(8):3374-81. doi: 10.1158/0008-5472.CAN-08-4290. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351827 Free PMC article.
-
Characterization of thrombin-bound dabigatran effects on protease-activated receptor-1 expression and signaling in vitro.Mol Pharmacol. 2015 Jul;88(1):95-105. doi: 10.1124/mol.114.096446. Epub 2015 May 1. Mol Pharmacol. 2015. PMID: 25934730 Free PMC article.
-
Thrombin induces protease-activated receptor 1 signaling and activation of human atrial fibroblasts and dabigatran prevents these effects.Int J Cardiol. 2018 Nov 15;271:219-227. doi: 10.1016/j.ijcard.2018.05.033. Epub 2018 May 24. Int J Cardiol. 2018. PMID: 29801760
-
Thrombin inhibition by dabigatran attenuates endothelial dysfunction in diabetic mice.Vascul Pharmacol. 2020 Jan;124:106632. doi: 10.1016/j.vph.2019.106632. Epub 2019 Nov 20. Vascul Pharmacol. 2020. PMID: 31759113
-
Thrombin inhibition and cyclophosphamide synergistically block tumor progression and metastasis.Cancer Biol Ther. 2015;16(12):1802-11. doi: 10.1080/15384047.2015.1078025. Cancer Biol Ther. 2015. PMID: 26383051 Free PMC article.
Cited by
-
Protease-activated receptor-1 (PAR-1): a promising molecular target for cancer.Oncotarget. 2017 Sep 18;8(63):107334-107345. doi: 10.18632/oncotarget.21015. eCollection 2017 Dec 5. Oncotarget. 2017. PMID: 29291033 Free PMC article. Review.
-
Commonly Prescribed Anticoagulants Exert Anticancer Effects in Oral Squamous Cell Carcinoma Cells In Vitro.Biology (Basel). 2022 Apr 14;11(4):596. doi: 10.3390/biology11040596. Biology (Basel). 2022. PMID: 35453795 Free PMC article.
-
A Novel Compound Targeting Protease Receptor 1 Activators for the Treatment of Glioblastoma.Front Neurol. 2018 Dec 17;9:1087. doi: 10.3389/fneur.2018.01087. eCollection 2018. Front Neurol. 2018. PMID: 30619047 Free PMC article.
-
Anti-Inflammatory and Anticancer Effects of Anticoagulant Therapy in Patients with Malignancy.Life (Basel). 2023 Sep 10;13(9):1888. doi: 10.3390/life13091888. Life (Basel). 2023. PMID: 37763292 Free PMC article. Review.
-
Downregulation of GRK5 hampers the migration of breast cancer cells.Sci Rep. 2019 Oct 29;9(1):15548. doi: 10.1038/s41598-019-51923-1. Sci Rep. 2019. PMID: 31664083 Free PMC article.
References
-
- Falanga, A. , Marchetti M., and Russo L.. 2015. The mechanisms of cancer‐associated thrombosis. Thromb. Res. 135(Suppl 1):S8–S11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

