Effect of tofacitinib withdrawal and re-treatment on patient-reported outcomes: results from a Phase 3 study in patients with moderate to severe chronic plaque psoriasis
- PMID: 27600367
- PMCID: PMC5297866
- DOI: 10.1111/jdv.13808
Effect of tofacitinib withdrawal and re-treatment on patient-reported outcomes: results from a Phase 3 study in patients with moderate to severe chronic plaque psoriasis
Abstract
Background: Tofacitinib is an oral Janus kinase inhibitor being investigated for psoriasis. A Phase 3 withdrawal/re-treatment study (NCT01186744; OPT Retreatment) showed tofacitinib re-treatment was effective in patients with chronic plaque psoriasis.
Objectives: To describe the effects of tofacitinib withdrawal/re-treatment on health-related quality of life (HRQoL) and disease symptoms measured by patient-reported outcomes (PROs).
Methods: The study was divided into initial treatment, treatment withdrawal, and re-treatment periods. Initial treatment: patients were randomized to receive tofacitinib 5 (n = 331) or 10 mg (n = 335) BID for 24 weeks. Treatment withdrawal: patients who achieved both ≥ 75% reduction in Psoriasis Area and Severity Index (PASI) score from baseline and Physician's Global Assessment of 'clear'/'almost clear' at Week (W)24 received placebo (withdrawal) or the previous dose (continuous treatment). Re-treatment: at relapse (> 50% loss of W24 PASI response) or at W40, patients received their initial tofacitinib dose. PROs included: Dermatology Life Quality Index (DLQI), Itch Severity Item (ISI), Short Form-36 (SF-36) and Patient's Global Assessment (PtGA).
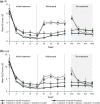
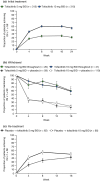
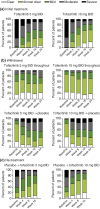
Results: After initial treatment with tofacitinib 5 and 10 mg BID, substantial and significant improvements were reported for mean DLQI (baseline: 12.6 and 12.6; W24: 5.1 and 2.6) and ISI (baseline: 6.7 and 6.9; W24: 2.9 and 1.6). Patients continuously treated with tofacitinib 5 and 10 mg BID maintained those improvements through Week 56 (DLQI: 3.0 and 2.1; ISI: 2.3 and 1.4). By W40, patients withdrawn from tofacitinib 5 and 10 mg BID showed worsening in DLQI (5.0 and 6.2) and ISI (3.7 and 4.0) scores; improvements were regained upon re-treatment (W56, DLQI: 3.4 and 2.4; ISI: 2.2 and 1.6). Similar results were reported for PtGA and SF-36.
Conclusion: Continuous tofacitinib treatment provided sustained improvement in HRQoL and disease symptoms. Patients randomized to treatment withdrawal lost initial improvements. Upon re-treatment, improvements were recaptured to levels comparable to those seen with continuous treatment.
© 2016 The Authors. Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Figures

References
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. - PubMed
-
- Reich A, Hrehorów E, Szepietowski JC. Pruritus is an important factor negatively influencing the well‐being of psoriatic patients. Acta Derm Venereol 2010; 90: 257–263. - PubMed
-
- Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. Am J Clin Dermatol 2005; 6: 383–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

