Salmonella Extracellular Matrix Components Influence Biofilm Formation and Gallbladder Colonization
- PMID: 27600501
- PMCID: PMC5067756
- DOI: 10.1128/IAI.00532-16
Salmonella Extracellular Matrix Components Influence Biofilm Formation and Gallbladder Colonization
Abstract
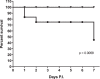
Salmonella enterica serovar Typhi, the causative agent of typhoid fever in humans, forms biofilms encapsulated by an extracellular matrix (ECM). Biofilms facilitate colonization and persistent infection in gallbladders of humans and mouse models of chronic carriage. Individual roles of matrix components have not been completely elucidated in vitro or in vivo To examine individual functions, strains of Salmonella enterica serovar Typhimurium, the murine model of S Typhi, in which various ECM genes were deleted or added, were created to examine biofilm formation, colonization, and persistence in the gallbladder. Studies show that curli contributes most significantly to biofilm formation. Expression of Vi antigen decreased biofilm formation in vitro and virulence and bacterial survival in vivo without altering the examined gallbladder pro- or anti-inflammatory cytokines. Oppositely, loss of all ECM components (ΔwcaM ΔcsgA ΔyihO ΔbcsE) increased virulence and bacterial survival in vivo and reduced gallbladder interleukin-10 (IL-10) levels. Colanic acid and curli mutants had the largest defects in biofilm-forming ability and contributed most significantly to the virulence increase of the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant strain. While the ΔwcaM ΔcsgA ΔyihO ΔbcsE mutant was not altered in resistance to complement or growth in macrophages, it attached and invaded macrophages better than the wild-type (WT) strain. These data suggest that ECM components have various levels of importance in biofilm formation and gallbladder colonization and that the ECM diminishes disseminated disease in our model, perhaps by reducing cell attachment/invasion and dampening inflammation by maintaining/inducing IL-10 production. Understanding how ECM components aid acute disease and persistence could lead to improvements in therapeutic treatment of typhoid fever patients.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures







Similar articles
-
A temporary cholesterol-rich diet and bacterial extracellular matrix factors favor Salmonella spp. biofilm formation in the cecum.mBio. 2025 Jan 8;16(1):e0324224. doi: 10.1128/mbio.03242-24. Epub 2024 Dec 5. mBio. 2025. PMID: 39636114 Free PMC article.
-
Pathoadaptive Alteration of Salmonella Biofilm Formation in Response to the Gallbladder Environment.J Bacteriol. 2019 Jun 21;201(14):e00774-18. doi: 10.1128/JB.00774-18. Print 2019 Jul 15. J Bacteriol. 2019. PMID: 30962351 Free PMC article.
-
Establishment of Chronic Typhoid Infection in a Mouse Carriage Model Involves a Type 2 Immune Shift and T and B Cell Recruitment to the Gallbladder.mBio. 2019 Oct 1;10(5):e02262-19. doi: 10.1128/mBio.02262-19. mBio. 2019. PMID: 31575775 Free PMC article.
-
Biofilm Producing Salmonella Typhi: Chronic Colonization and Development of Gallbladder Cancer.Int J Mol Sci. 2017 Aug 31;18(9):1887. doi: 10.3390/ijms18091887. Int J Mol Sci. 2017. PMID: 28858232 Free PMC article. Review.
-
Regulation of biofilm formation in Salmonella enterica serovar Typhimurium.Future Microbiol. 2014;9(11):1261-82. doi: 10.2217/fmb.14.88. Future Microbiol. 2014. PMID: 25437188 Review.
Cited by
-
The Rcs-Regulated Colanic Acid Capsule Maintains Membrane Potential in Salmonella enterica serovar Typhimurium.mBio. 2017 Jun 6;8(3):e00808-17. doi: 10.1128/mBio.00808-17. mBio. 2017. PMID: 28588134 Free PMC article.
-
Characterization of Invasive Salmonella Serogroup C1 Infections in Mali.Am J Trop Med Hyg. 2018 Feb;98(2):589-594. doi: 10.4269/ajtmh.17-0508. Epub 2017 Dec 21. Am J Trop Med Hyg. 2018. PMID: 29280425 Free PMC article.
-
Salmonella Biofilms Tolerate Hydrogen Peroxide by a Combination of Extracellular Polymeric Substance Barrier Function and Catalase Enzymes.Front Cell Infect Microbiol. 2021 May 19;11:683081. doi: 10.3389/fcimb.2021.683081. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34095002 Free PMC article.
-
Abundant production of exopolysaccharide by EAEC strains enhances the formation of bacterial biofilms in contaminated sprouts.Gut Microbes. 2018;9(3):264-278. doi: 10.1080/19490976.2018.1429877. Epub 2018 Apr 19. Gut Microbes. 2018. PMID: 29543544 Free PMC article.
-
The Escherichia coli cellulose synthase subunit G (BcsG) is a Zn2+-dependent phosphoethanolamine transferase.J Biol Chem. 2020 May 1;295(18):6225-6235. doi: 10.1074/jbc.RA119.011668. Epub 2020 Mar 9. J Biol Chem. 2020. PMID: 32152228 Free PMC article.
References
-
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. 2015. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. doi:10.1371/journal.pmed.1001921. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

