Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation
- PMID: 27600536
- PMCID: PMC5059793
- DOI: 10.1105/tpc.16.00124
Morphogenic Regulators Baby boom and Wuschel Improve Monocot Transformation
Abstract
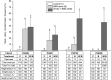
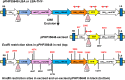
While transformation of the major monocot crops is currently possible, the process typically remains confined to one or two genotypes per species, often with poor agronomics, and efficiencies that place these methods beyond the reach of most academic laboratories. Here, we report a transformation approach involving overexpression of the maize (Zea mays) Baby boom (Bbm) and maize Wuschel2 (Wus2) genes, which produced high transformation frequencies in numerous previously nontransformable maize inbred lines. For example, the Pioneer inbred PHH5G is recalcitrant to biolistic and Agrobacterium tumefaciens transformation. However, when Bbm and Wus2 were expressed, transgenic calli were recovered from over 40% of the starting explants, with most producing healthy, fertile plants. Another limitation for many monocots is the intensive labor and greenhouse space required to supply immature embryos for transformation. This problem could be alleviated using alternative target tissues that could be supplied consistently with automated preparation. As a major step toward this objective, we transformed Bbm and Wus2 directly into either embryo slices from mature seed or leaf segments from seedlings in a variety of Pioneer inbred lines, routinely recovering healthy, fertile T0 plants. Finally, we demonstrated that the maize Bbm and Wus2 genes stimulate transformation in sorghum (Sorghum bicolor) immature embryos, sugarcane (Saccharum officinarum) callus, and indica rice (Oryza sativa ssp indica) callus.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures



Similar articles
-
Maize Transformation Using the Morphogenic Genes Baby Boom and Wuschel2.Methods Mol Biol. 2019;1864:81-93. doi: 10.1007/978-1-4939-8778-8_6. Methods Mol Biol. 2019. PMID: 30415330
-
Transformation of Recalcitrant Sorghum Varieties Facilitated by Baby Boom and Wuschel2.Curr Protoc Plant Biol. 2018 Dec;3(4):e20076. doi: 10.1002/cppb.20076. Epub 2018 Oct 17. Curr Protoc Plant Biol. 2018. PMID: 30369099
-
Rapid genotype "independent" Zea mays L. (maize) transformation via direct somatic embryogenesis.In Vitro Cell Dev Biol Plant. 2018;54(3):240-252. doi: 10.1007/s11627-018-9905-2. Epub 2018 Apr 30. In Vitro Cell Dev Biol Plant. 2018. PMID: 29780216 Free PMC article.
-
Unlocking regeneration potential: harnessing morphogenic regulators and small peptides for enhanced plant engineering.Plant J. 2025 Jan;121(2):e17193. doi: 10.1111/tpj.17193. Epub 2024 Dec 10. Plant J. 2025. PMID: 39658544 Free PMC article. Review.
-
Application of Wox2a in transformation of recalcitrant maize genotypes.aBIOTECH. 2023 Oct 13;4(4):386-388. doi: 10.1007/s42994-023-00116-6. eCollection 2023 Dec. aBIOTECH. 2023. PMID: 38106431 Free PMC article. Review.
Cited by
-
Nanotechnology to advance CRISPR-Cas genetic engineering of plants.Nat Nanotechnol. 2021 Mar;16(3):243-250. doi: 10.1038/s41565-021-00854-y. Epub 2021 Mar 12. Nat Nanotechnol. 2021. PMID: 33712738 Free PMC article. Review.
-
From Traditional Breeding to Genome Editing for Boosting Productivity of the Ancient Grain Tef [Eragrostis tef (Zucc.) Trotter].Plants (Basel). 2021 Mar 25;10(4):628. doi: 10.3390/plants10040628. Plants (Basel). 2021. PMID: 33806233 Free PMC article.
-
Genetic resources and precise gene editing for targeted improvement of barley abiotic stress tolerance.J Zhejiang Univ Sci B. 2023 Jul 3;24(12):1069-1092. doi: 10.1631/jzus.B2200552. J Zhejiang Univ Sci B. 2023. PMID: 38057266 Free PMC article. Review.
-
Trichostatin A Triggers an Embryogenic Transition in Arabidopsis Explants via an Auxin-Related Pathway.Front Plant Sci. 2018 Sep 13;9:1353. doi: 10.3389/fpls.2018.01353. eCollection 2018. Front Plant Sci. 2018. PMID: 30271420 Free PMC article.
-
Genetic Transformation of Apomictic Grasses: Progress and Constraints.Front Plant Sci. 2021 Nov 5;12:768393. doi: 10.3389/fpls.2021.768393. eCollection 2021. Front Plant Sci. 2021. PMID: 34804102 Free PMC article. Review.
References
-
- Ahmadabadi M., Ruf S., Bock R. (2007). A leaf-based regeneration and transformation system for maize (Zea mays L.). Transgenic Res. 16: 437–448. - PubMed
-
- Al-Abed D., Rudrabhatla S., Talla R., Goldman S. (2006). Split-seed: a new tool for maize researchers. Planta 223: 1355–1360. - PubMed
-
- Ananiev E.V., Wu C., Chamberlin M.A., Svitashev S., Schwartz C., Gordon-Kamm W., Tingey S. (2009). Artificial chromosome formation in maize (Zea mays L.). Chromosoma 118: 157–177. - PubMed
-
- Armstrong C.L., Green C.E. (1985). Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 164: 207–214. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

