An NADPH-Oxidase/Polyamine Oxidase Feedback Loop Controls Oxidative Burst Under Salinity
- PMID: 27600815
- PMCID: PMC5100782
- DOI: 10.1104/pp.16.01118
An NADPH-Oxidase/Polyamine Oxidase Feedback Loop Controls Oxidative Burst Under Salinity
Abstract
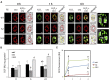
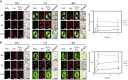
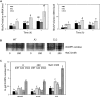
The apoplastic polyamine oxidase (PAO) catalyzes the oxidation of the higher polyamines spermidine and spermine, contributing to hydrogen peroxide (H2O2) accumulation. However, it is yet unclear whether apoplastic PAO is part of a network that coordinates the accumulation of reactive oxygen species (ROS) under salinity or if it acts independently. Here, we unravel that NADPH oxidase and apoplastic PAO cooperate to control the accumulation of H2O2 and superoxides (O2·-) in tobacco (Nicotiana tabacum). To examine to what extent apoplastic PAO constitutes part of a ROS-generating network, we examined ROS accumulation in guard cells of plants overexpressing or down-regulating apoplastic PAO (lines S2.2 and A2, respectively) or down-regulating NADPH oxidase (line AS-NtRbohD/F). The H2O2-specific probe benzene sulfonyl-H2O2 showed that, under salinity, H2O2 increased in S2.2 and decreased in A2 compared with the wild type. Surprisingly, the O2·--specific probe benzene sulfonyl-So showed that O2·- levels correlated positively with that of apoplastic PAO (i.e. showed high and low levels in S2.2 and A2, respectively). By using AS-NtRbohD/F lines and a pharmacological approach, we could show that H2O2 and O2·- accumulation at the onset of salinity stress was dependent on NADPH oxidase, indicating that NADPH oxidase is upstream of apoplastic PAO. Our results suggest that NADPH oxidase and the apoplastic PAO form a feed-forward ROS amplification loop, which impinges on oxidative state and culminates in the execution of programmed cell death. We propose that the PAO/NADPH oxidase loop is a central hub in the plethora of responses controlling salt stress tolerance, with potential functions extending beyond stress tolerance.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

