Deimmunization for gene therapy: host matching of synthetic zinc finger constructs enables long-term mutant Huntingtin repression in mice
- PMID: 27600816
- PMCID: PMC5013590
- DOI: 10.1186/s13024-016-0128-x
Deimmunization for gene therapy: host matching of synthetic zinc finger constructs enables long-term mutant Huntingtin repression in mice
Abstract
Background: Synthetic zinc finger (ZF) proteins can be targeted to desired DNA sequences and are useful tools for gene therapy. We recently developed a ZF transcription repressor (ZF-KOX1) able to bind to expanded DNA CAG-repeats in the huntingtin (HTT) gene, which are found in Huntington's disease (HD). This ZF acutely repressed mutant HTT expression in a mouse model of HD and delayed neurological symptoms (clasping) for up to 3 weeks. In the present work, we sought to develop a long-term single-injection gene therapy approach in the brain.
Method: Since non-self proteins can elicit immune and inflammatory responses, we designed a host-matched analogue of ZF-KOX1 (called mZF-KRAB), to treat mice more safely in combination with rAAV vector delivery. We also tested a neuron-specific enolase promoter (pNSE), which has been reported as enabling long-term transgene expression, to see whether HTT repression could be observed for up to 6 months after AAV injection in the brain.
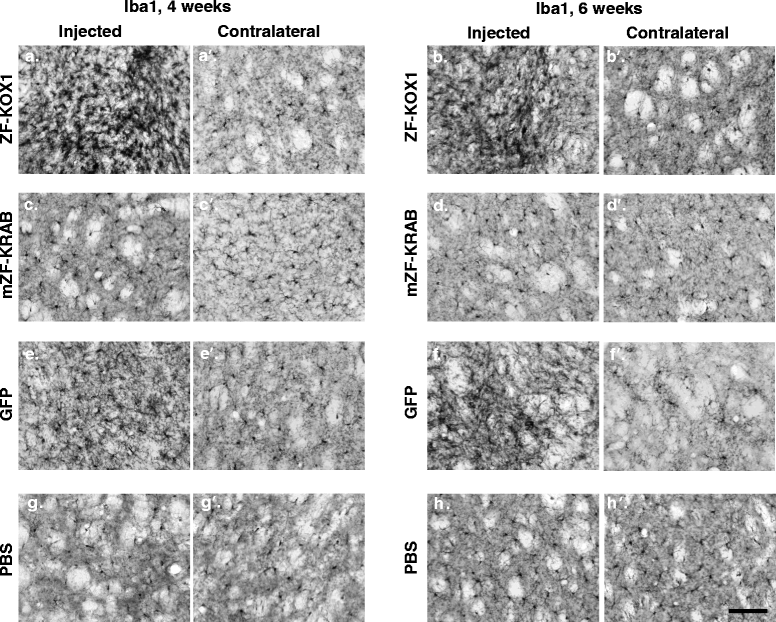
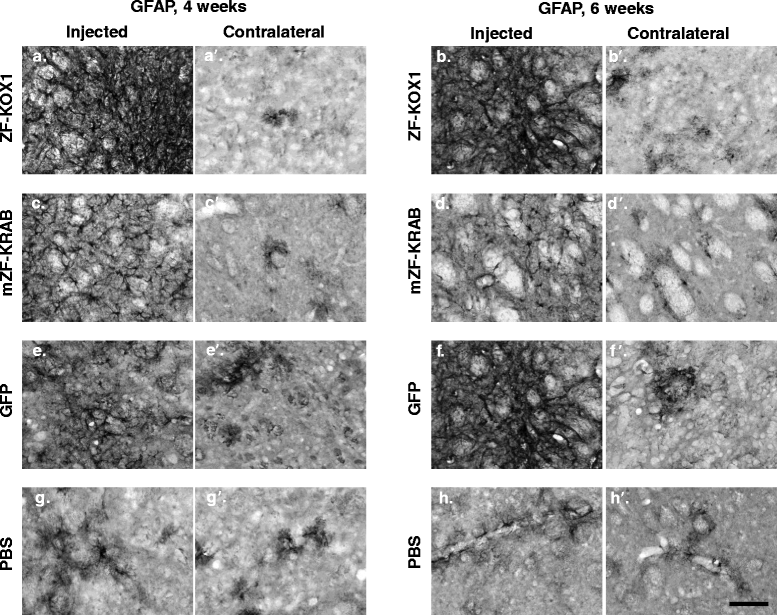
Results: After rAAV vector delivery, we found that non-self proteins induce significant inflammatory responses in the brain, in agreement with previous studies. Specifically, microglial cells were activated at 4 and 6 weeks after treatment with non-host-matched ZF-KOX1 or GFP, respectively, and this was accompanied by a moderate neuronal loss. In contrast, the host-matched mZF-KRAB did not provoke these effects. Nonetheless, we found that using a pCAG promoter (CMV early enhancer element and the chicken β-actin promoter) led to a strong reduction in ZF expression by 6 weeks after injection. We therefore tested a new non-viral promoter to see whether the host-adapted ZF expression could be sustained for a longer time. Vectorising mZF-KRAB with a promoter-enhancer from neuron-specific enolase (Eno2, rat) resulted in up to 77 % repression of mutant HTT in whole brain, 3 weeks after bilateral intraventricular injection of 10(10) virions. Importantly, repressions of 48 % and 23 % were still detected after 12 and 24 weeks, respectively, indicating that longer term effects are possible.
Conclusion: Host-adapted ZF-AAV constructs displayed a reduced toxicity and a non-viral pNSE promoter improved long-term ZF protein expression and target gene repression. The optimized constructs presented here have potential for treating HD.
Keywords: Gene therapy; Host optimization; Huntington’s disease; Immune response; Monogenetic disease; Neurodegenerative disorder; Synthetic transcription factors; rAAV.
Figures






References
-
- Yin D, Zhai Y, Gruber HE, Ibanez CE, Robbins JM, Kells a P, et al. Convection-enhanced delivery improves distribution and efficacy of tumor-selective retroviral replicating vectors in a rodent brain tumor model. Cancer Gene Ther [Internet] 2013;20:336–41. doi: 10.1038/cgt.2013.25. - DOI - PMC - PubMed
-
- Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science [Internet] 2013;341:1233158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

