Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial
- PMID: 27600829
- PMCID: PMC5012017
- DOI: 10.1186/s13075-016-1096-9
Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial
Abstract
Background: Sarilumab is a human monoclonal antibody directed against the alpha subunit of the interleukin-6 receptor complex. In the MOBILITY phase III randomized controlled trial (RCT), sarilumab + methotrexate (MTX) treatment resulted in clinical improvements at 24 weeks that were maintained at 52 weeks in adults with rheumatoid arthritis (RA), who have inadequate response to MTX (MTX-IR). These analyses indicate the effects of sarilumab + MTX versus placebo on patient-reported outcomes (PROs) in this RCT.
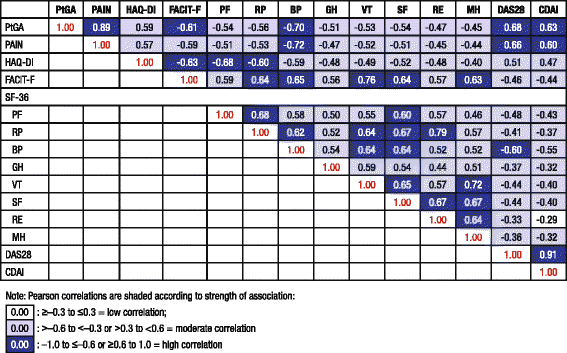
Methods: Patients (n = 1197) were randomized to receive placebo, sarilumab 150 or 200 mg subcutaneously + MTX every 2 weeks for 52 weeks; after 16 weeks, patients without ≥20 % improvement from baseline in swollen or tender joint counts on two consecutive assessments were offered open-label treatment. PROs included patient global assessment of disease activity (PtGA), pain, health assessment questionnaire disability index (HAQ-DI), Short Form-36 Health Survey (SF-36), and functional assessment of chronic illness therapy-fatigue (FACIT-F). Changes from baseline at weeks 24 and 52 were analyzed using a mixed model for repeated measures. Post hoc analyses included percentages of patients reporting improvements equal to or greater than minimal clinically important differences (MCID) and normative values in the FACIT-F and SF-36. Pearson correlation between observed PRO scores and clinical measures of disease activity was tested at week 24.
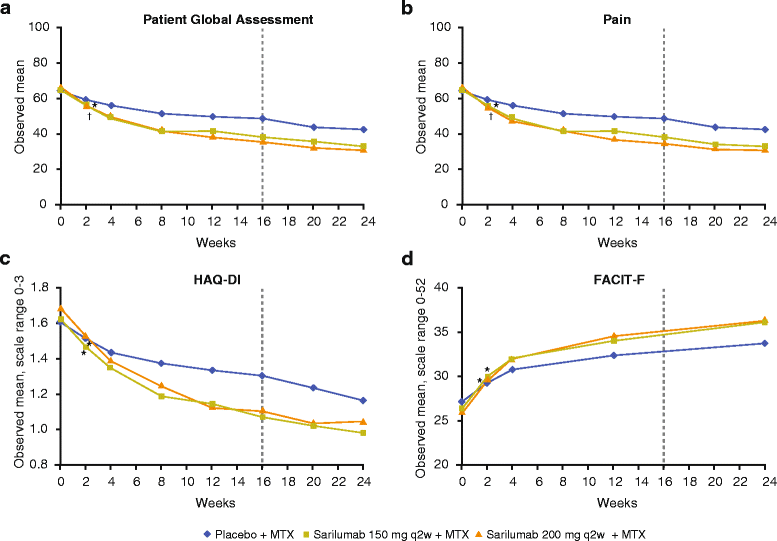
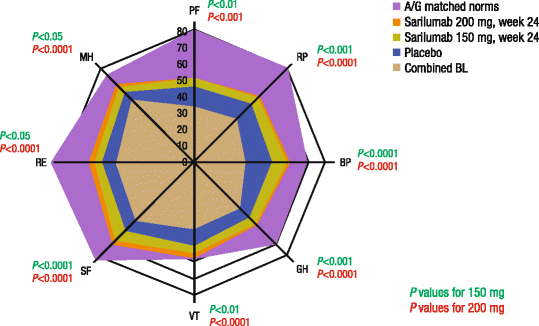
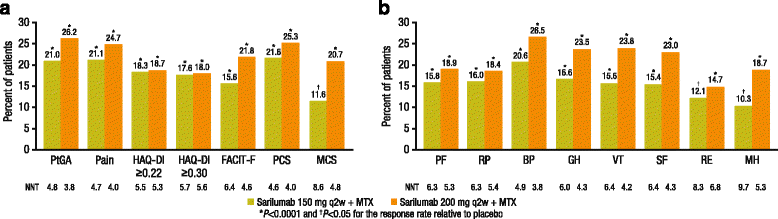
Results: Both doses of sarilumab + MTX vs placebo + MTX resulted in improvement from baseline by week 24 in PtGA, pain, HAQ-DI, SF-36 and FACIT-F scores (p < 0.0001) that was clinically meaningful, and persisted until week 52. In post hoc analyses, the percentages of patients with improvement equal to or greater than the MCID across all PROs were greater with sarilumab than placebo (p < 0.05), with differences ranging from 11.6 to 26.2 %, as were those reporting equal to or greater than normative scores.
Conclusions: In this RCT in patients with MTX-IR RA, sarilumab + MTX resulted in sustained improvement in PROs that were clinically meaningful, greater than placebo + MTX, and complement the previously reported clinical efficacy and safety of sarilumab.
Trial registration: ClinicalTrials.gov. NCT01061736 . February 2, 2010.
Keywords: Fatigue; Interleukin-6; Patient-reported outcomes; Rheumatoid arthritis; Sarilumab.
Figures

References
-
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. The Committee on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Arthritis Rheum. 1993;36(6):729–40. doi: 10.1002/art.1780360601. - DOI - PubMed
-
- Kirwan JR, Hewlett SE, Heiberg T, Hughes RA, Carr M, Hehir M, et al. Incorporating the patient perspective into outcome assessment in rheumatoid arthritis–progress at OMERACT 7. J Rheumatol. 2005;32(11):2250–6. - PubMed
-
- Genovese MC, Fleischmann R, Kivitz AJ, Rell-Bakalarska M, Martincova R, Fiore S, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III Study. Arthritis Rheumatol. 2015;67(6):1424–37. doi: 10.1002/art.39093. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

