Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly
- PMID: 27601476
- PMCID: PMC5077203
- DOI: 10.1074/jbc.M116.739573
Arginine Demethylation of G3BP1 Promotes Stress Granule Assembly
Abstract
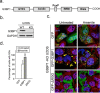
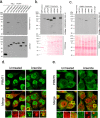
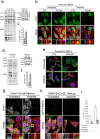
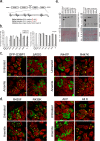
Stress granules (SGs) are cytoplasmic condensates of stalled messenger ribonucleoprotein complexes (mRNPs) that form when eukaryotic cells encounter environmental stress. RNA-binding proteins are enriched for arginine methylation and facilitate SG assembly through interactions involving regions of low amino acid complexity. How methylation of specific RNA-binding proteins regulates RNA granule assembly has not been characterized. Here, we examined the potent SG-nucleating protein Ras-GAP SH3-binding protein 1 (G3BP1), and found that G3BP1 is differentially methylated on specific arginine residues by protein arginine methyltransferase (PRMT) 1 and PRMT5 in its RGG domain. Several genetic and biochemical interventions that increased methylation repressed SG assembly, whereas interventions that decreased methylation promoted SG assembly. Arsenite stress quickly and reversibly decreased asymmetric arginine methylation on G3BP1. These data indicate that arginine methylation in the RGG domain prevents large SG assembly and rapid demethylation is a novel signal that regulates SG formation.
Keywords: G3BP1; RNA binding protein; mRNA; post-translational modification (PTM); protein arginine N-methyltransferase (PRMT); protein arginine N-methyltransferase 5 (PRMT5); stress granule.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

