Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer
- PMID: 27601552
- PMCID: PMC5477982
- DOI: 10.1200/JCO.2016.68.1239
Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer
Abstract
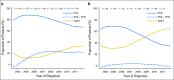
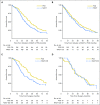
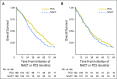
Purpose In 2010, a randomized clinical trial demonstrated noninferior survival for patients with advanced ovarian cancer who were treated with neoadjuvant chemotherapy (NACT) compared with primary cytoreductive surgery (PCS). We examined the use and effectiveness of NACT in clinical practice. Patients and Methods A multi-institutional observational study of 1,538 women with stages IIIC to IV ovarian cancer who were treated at six National Cancer Institute-designated cancer centers. We examined NACT use in patients who were diagnosed between 2003 and 2012 (N = 1,538) and compared overall survival (OS), morbidity, and postoperative residual disease in a propensity-score matched sample of patients (N = 594). Results NACT use increased from 16% during 2003 to 2010 to 34% during 2011 to 2012 in stage IIIC disease ( Ptrend < .001), and from 41% to 62% in stage IV disease ( Ptrend < .001). Adoption of NACT varied by institution, from 8% to 30% for stage IIIC disease (P < .001) and from 27% to 61% ( P = .007) for stage IV disease during this time period. In the matched sample, NACT was associated with shorter OS in stage IIIC disease (median OS: 33 v 43 months; hazard ratio [HR], 1.40; 95% CI, 1.11 to 1.77) compared with PCS, but not stage IV disease (median OS: 31 v 36 months; HR, 1.16; 95% CI, 0.89 to 1.52). Patients with stages IIIC and IV disease who received NACT were less likely to have ≥ 1 cm postoperative residual disease, an intensive care unit admission, or a rehospitalization (all P ≤ .04) compared with those who received PCS treatment. However, among women with stage IIIC disease who achieved microscopic or ≤ 1 cm postoperative residual disease, NACT was associated with decreased OS (HR, 1.49; 95% CI, 1.01 to 2.18; P = .04). Conclusion Use of NACT increased significantly between 2003 and 2012. In this observational study, PCS was associated with increased survival in stage IIIC, but not stage IV disease. Future studies should prospectively consider the efficacy of NACT by extent of residual disease in unselected patients.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
How to Select Neoadjuvant Chemotherapy or Primary Debulking Surgery in Patients With Stage IIIC or IV Ovarian Carcinoma.J Clin Oncol. 2016 Nov 10;34(32):3827-3828. doi: 10.1200/JCO.2016.69.7458. Epub 2016 Sep 30. J Clin Oncol. 2016. PMID: 27646940 No abstract available.
-
Neoadjuvant Chemotherapy or Primary Debulking Surgery for Stage IIIC Ovarian Cancer.J Clin Oncol. 2017 Mar;35(7):802-803. doi: 10.1200/JCO.2016.70.7125. Epub 2016 Dec 28. J Clin Oncol. 2017. PMID: 28029302 No abstract available.
-
Reply to M.S. Copur et al.J Clin Oncol. 2017 Mar;35(7):803. doi: 10.1200/JCO.2016.71.1978. Epub 2016 Dec 28. J Clin Oncol. 2017. PMID: 28029325 No abstract available.
References
-
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. - PubMed
-
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. - PubMed
-
- Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol Oncol. 2006;103:1070–1076. - PubMed
-
- Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–2320. - PubMed
-
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

