Axoneme Structure from Motile Cilia
- PMID: 27601632
- PMCID: PMC5204319
- DOI: 10.1101/cshperspect.a028076
Axoneme Structure from Motile Cilia
Abstract
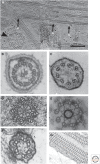
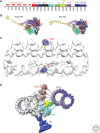
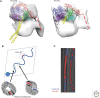
The axoneme is the main extracellular part of cilia and flagella in eukaryotes. It consists of a microtubule cytoskeleton, which normally comprises nine doublets. In motile cilia, dynein ATPase motor proteins generate sliding motions between adjacent microtubules, which are integrated into a well-orchestrated beating or rotational motion. In primary cilia, there are a number of sensory proteins functioning on membranes surrounding the axoneme. In both cases, as the study of proteomics has elucidated, hundreds of proteins exist in this compartmentalized biomolecular system. In this article, we review the recent progress of structural studies of the axoneme and its components using electron microscopy and X-ray crystallography, mainly focusing on motile cilia. Structural biology presents snapshots (but not live imaging) of dynamic structural change and gives insights into the force generation mechanism of dynein, ciliary bending mechanism, ciliogenesis, and evolution of the axoneme.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
