Minibrain and Wings apart control organ growth and tissue patterning through down-regulation of Capicua
- PMID: 27601662
- PMCID: PMC5035877
- DOI: 10.1073/pnas.1609417113
Minibrain and Wings apart control organ growth and tissue patterning through down-regulation of Capicua
Abstract
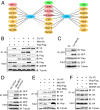
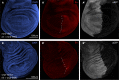
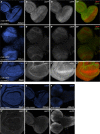
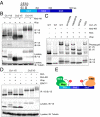
The transcriptional repressor Capicua (Cic) controls tissue patterning and restricts organ growth, and has been recently implicated in several cancers. Cic has emerged as a primary sensor of signaling downstream of the receptor tyrosine kinase (RTK)/extracellular signal-regulated kinase (ERK) pathway, but how Cic activity is regulated in different cellular contexts remains poorly understood. We found that the kinase Minibrain (Mnb, ortholog of mammalian DYRK1A), acting through the adaptor protein Wings apart (Wap), physically interacts with and phosphorylates the Cic protein. Mnb and Wap inhibit Cic function by limiting its transcriptional repressor activity. Down-regulation of Cic by Mnb/Wap is necessary for promoting the growth of multiple organs, including the wings, eyes, and the brain, and for proper tissue patterning in the wing. We have thus uncovered a previously unknown mechanism of down-regulation of Cic activity by Mnb and Wap, which operates independently from the ERK-mediated control of Cic. Therefore, Cic functions as an integrator of upstream signals that are essential for tissue patterning and organ growth. Finally, because DYRK1A and CIC exhibit, respectively, prooncogenic vs. tumor suppressor activities in human oligodendroglioma, our results raise the possibility that DYRK1A may also down-regulate CIC in human cells.
Keywords: DYRK1A; capicua; minibrain; organ growth; tissue patterning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

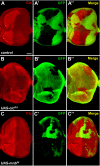

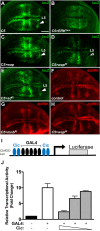


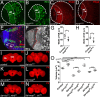
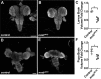

References
-
- Goff DJ, Nilson LA, Morisato D. Establishment of dorsal-ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development. 2001;128(22):4553–4562. - PubMed
-
- Roch F, Jiménez G, Casanova J. EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development. 2002;129(4):993–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

