TS-EUROTRAIN: A European-Wide Investigation and Training Network on the Etiology and Pathophysiology of Gilles de la Tourette Syndrome
- PMID: 27601976
- PMCID: PMC4994475
- DOI: 10.3389/fnins.2016.00384
TS-EUROTRAIN: A European-Wide Investigation and Training Network on the Etiology and Pathophysiology of Gilles de la Tourette Syndrome
Abstract
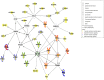
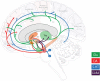
Gilles de la Tourette Syndrome (GTS) is characterized by the presence of multiple motor and phonic tics with a fluctuating course of intensity, frequency, and severity. Up to 90% of patients with GTS present with comorbid conditions, most commonly attention-deficit/hyperactivity disorder (ADHD), and obsessive-compulsive disorder (OCD), thus providing an excellent model for the exploration of shared etiology across disorders. TS-EUROTRAIN (FP7-PEOPLE-2012-ITN, Grant Agr.No. 316978) is a Marie Curie Initial Training Network (http://ts-eurotrain.eu) that aims to elucidate the complex etiology of the onset and clinical course of GTS, investigate the neurobiological underpinnings of GTS and related disorders, translate research findings into clinical applications, and establish a pan-European infrastructure for the study of GTS. This includes the challenges of (i) assembling a large genetic database for the evaluation of the genetic architecture with high statistical power; (ii) exploring the role of gene-environment interactions including the effects of epigenetic phenomena; (iii) employing endophenotype-based approaches to understand the shared etiology between GTS, OCD, and ADHD; (iv) establishing a developmental animal model for GTS; (v) gaining new insights into the neurobiological mechanisms of GTS via cross-sectional and longitudinal neuroimaging studies; and (vi) partaking in outreach activities including the dissemination of scientific knowledge about GTS to the public. Fifteen partners from academia and industry and 12 PhD candidates pursue the project. Here, we aim to share the design of an interdisciplinary project, showcasing the potential of large-scale collaborative efforts in the field of GTS. Our ultimate aims are to elucidate the complex etiology and neurobiological underpinnings of GTS, translate research findings into clinical applications, and establish Pan-European infrastructure for the study of GTS and associated disorders.
Keywords: Gilles de la Tourette Syndrome; Initial Training Network; animal models; etiology; genetics; neuroimaging; tourette disorder.
Figures
References
-
- American Psychiatric Association (2013). Neurodevelopmental Disorders, in Diagnostic and Statistical Manual of Mental Disorders DSM Library (Washington, DC: American Psychiatric Association; ), 81–82.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

