Correlative Light Electron Microscopy: Connecting Synaptic Structure and Function
- PMID: 27601992
- PMCID: PMC4993758
- DOI: 10.3389/fnsyn.2016.00028
Correlative Light Electron Microscopy: Connecting Synaptic Structure and Function
Abstract
Many core paradigms of contemporary neuroscience are based on information obtained by electron or light microscopy. Intriguingly, these two imaging techniques are often viewed as complementary, yet separate entities. Recent technological advancements in microscopy techniques, labeling tools, and fixation or preparation procedures have fueled the development of a series of hybrid approaches that allow correlating functional fluorescence microscopy data and ultrastructural information from electron micrographs from a singular biological event. As correlative light electron microscopy (CLEM) approaches become increasingly accessible, long-standing neurobiological questions regarding structure-function relation are being revisited. In this review, we will survey what developments in electron and light microscopy have spurred the advent of correlative approaches, highlight the most relevant CLEM techniques that are currently available, and discuss its potential and limitations with respect to neuronal and synapse-specific applications.
Keywords: CLEM; SEM; TEM; correlative light electron microscopy; electron microscopy; fluorescence microscopy; neuron; synapse.
Figures
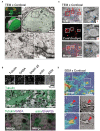

References
-
- Abbe (1883). The relation of aperture and power in the microscope. J. R. Microsc. Soc. 3 790–812. 10.1111/j.1365-2818.1883.tb05956.x - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

