The Regulatory Protein RosR Affects Rhizobium leguminosarum bv. trifolii Protein Profiles, Cell Surface Properties, and Symbiosis with Clover
- PMID: 27602024
- PMCID: PMC4993760
- DOI: 10.3389/fmicb.2016.01302
The Regulatory Protein RosR Affects Rhizobium leguminosarum bv. trifolii Protein Profiles, Cell Surface Properties, and Symbiosis with Clover
Abstract
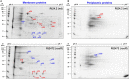
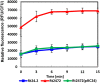
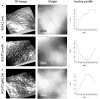
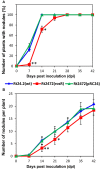
Rhizobium leguminosarum bv. trifolii is capable of establishing a symbiotic relationship with plants from the genus Trifolium. Previously, a regulatory protein encoded by rosR was identified and characterized in this bacterium. RosR possesses a Cys2-His2-type zinc finger motif and belongs to Ros/MucR family of rhizobial transcriptional regulators. Transcriptome profiling of the rosR mutant revealed a role of this protein in several cellular processes, including the synthesis of cell-surface components and polysaccharides, motility, and bacterial metabolism. Here, we show that a mutation in rosR resulted in considerable changes in R. leguminosarum bv. trifolii protein profiles. Extracellular, membrane, and periplasmic protein profiles of R. leguminosarum bv. trifolii wild type and the rosR mutant were examined, and proteins with substantially different abundances between these strains were identified. Compared with the wild type, extracellular fraction of the rosR mutant contained greater amounts of several proteins, including Ca(2+)-binding cadherin-like proteins, a RTX-like protein, autoaggregation protein RapA1, and flagellins FlaA and FlaB. In contrast, several proteins involved in the uptake of various substrates were less abundant in the mutant strain (DppA, BraC, and SfuA). In addition, differences were observed in membrane proteins of the mutant and wild-type strains, which mainly concerned various transport system components. Using atomic force microscopy (AFM) imaging, we characterized the topography and surface properties of the rosR mutant and wild-type cells. We found that the mutation in rosR gene also affected surface properties of R. leguminosarum bv. trifolii. The mutant cells were significantly more hydrophobic than the wild-type cells, and their outer membrane was three times more permeable to the hydrophobic dye N-phenyl-1-naphthylamine. The mutation of rosR also caused defects in bacterial symbiotic interaction with clover plants. Compared with the wild type, the rosR mutant infected host plant roots much less effectively and its nodule occupation was disturbed. At the ultrastructural level, the most striking differences between the mutant and the wild-type nodules concerned the structure of infection threads, release of bacteria, and bacteroid differentiation. This confirms an essential role of RosR in establishment of successful symbiotic interaction of R. leguminosarum bv. trifolii with clover plants.
Keywords: Rhizobium leguminosarum; clover; envelope properties; extracellular proteins; membrane proteins; rosR gene; symbiosis.
Figures




Similar articles
-
Transcriptome profiling of a Rhizobium leguminosarum bv. trifolii rosR mutant reveals the role of the transcriptional regulator RosR in motility, synthesis of cell-surface components, and other cellular processes.BMC Genomics. 2015 Dec 29;16:1111. doi: 10.1186/s12864-015-2332-4. BMC Genomics. 2015. PMID: 26715155 Free PMC article.
-
Rhizobium leguminosarum bv. trifolii rosR is required for interaction with clover, biofilm formation and adaptation to the environment.BMC Microbiol. 2010 Nov 11;10:284. doi: 10.1186/1471-2180-10-284. BMC Microbiol. 2010. PMID: 21070666 Free PMC article.
-
The Rhizobium leguminosarum bv. trifolii RosR: transcriptional regulator involved in exopolysaccharide production.Mol Plant Microbe Interact. 2007 Jul;20(7):867-81. doi: 10.1094/MPMI-20-7-0867. Mol Plant Microbe Interact. 2007. PMID: 17601173
-
Modulation of rosR expression and exopolysaccharide production in Rhizobium leguminosarum bv. trifolii by phosphate and clover root exudates.Int J Mol Sci. 2011;12(6):4132-55. doi: 10.3390/ijms12064132. Epub 2011 Jun 22. Int J Mol Sci. 2011. PMID: 21747729 Free PMC article.
-
Multiple copies of rosR and pssA genes enhance exopolysaccharide production, symbiotic competitiveness and clover nodulation in Rhizobium leguminosarum bv. trifolii.Antonie Van Leeuwenhoek. 2009 Nov;96(4):471-86. doi: 10.1007/s10482-009-9362-3. Epub 2009 Jul 9. Antonie Van Leeuwenhoek. 2009. PMID: 19588265
Cited by
-
Competition, Nodule Occupancy, and Persistence of Inoculant Strains: Key Factors in the Rhizobium-Legume Symbioses.Front Plant Sci. 2021 Aug 19;12:690567. doi: 10.3389/fpls.2021.690567. eCollection 2021. Front Plant Sci. 2021. PMID: 34489993 Free PMC article. Review.
-
Mutation in the pssZ Gene Negatively Impacts Exopolysaccharide Synthesis, Surface Properties, and Symbiosis of Rhizobium leguminosarum bv. trifolii with Clover.Genes (Basel). 2018 Jul 23;9(7):369. doi: 10.3390/genes9070369. Genes (Basel). 2018. PMID: 30041474 Free PMC article.
-
Rhizobial Exopolysaccharides: Genetic Regulation of Their Synthesis and Relevance in Symbiosis with Legumes.Int J Mol Sci. 2021 Jun 9;22(12):6233. doi: 10.3390/ijms22126233. Int J Mol Sci. 2021. PMID: 34207734 Free PMC article. Review.
-
Structure and Development of the Legume-Rhizobial Symbiotic Interface in Infection Threads.Cells. 2021 Apr 29;10(5):1050. doi: 10.3390/cells10051050. Cells. 2021. PMID: 33946779 Free PMC article. Review.
-
A Mutation in the Mesorhizobium loti oatB Gene Alters the Physicochemical Properties of the Bacterial Cell Wall and Reduces Survival inside Acanthamoeba castellanii.Int J Mol Sci. 2018 Nov 8;19(11):3510. doi: 10.3390/ijms19113510. Int J Mol Sci. 2018. PMID: 30413017 Free PMC article.
References
-
- Abdian P. L., Caramelo J. J., Ausmees N., Zorreguieta A. (2012). RapA2 is a calcium-binding lectin composed of two highly conserved cadherin-like domains that specifically recognize Rhizobium leguminosarum acidic exopolysaccharides. J. Biol. Chem. 288, 2893–2904. 10.1074/jbc.M112.411769 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

