Molecular Evolution and Phylogeography of Co-circulating IHNV and VHSV in Italy
- PMID: 27602026
- PMCID: PMC4994472
- DOI: 10.3389/fmicb.2016.01306
Molecular Evolution and Phylogeography of Co-circulating IHNV and VHSV in Italy
Abstract
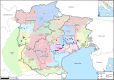
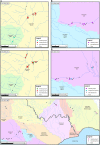
Infectious haematopoietic necrosis virus (IHNV) and viral haemorrhagic septicaemia virus (VHSV) are the most important viral pathogens impacting rainbow trout farming. These viruses are persistent in Italy, where they are responsible for severe disease outbreaks (epizootics) that affect the profitability of the trout industry. Despite the importance of IHNV and VHSV, little is known about their evolution at a local scale, although this is likely to be important for virus eradication and control. To address this issue we performed a detailed molecular evolutionary and epidemiological analysis of IHNV and VHSV in trout farms from northern Italy. Full-length glycoprotein gene sequences of a selection of VHSV (n = 108) and IHNV (n = 89) strains were obtained. This revealed that Italian VHSV strains belong to sublineages Ia1 and Ia2 of genotype Ia and are distributed into 7 genetic clusters. In contrast, all Italian IHNV isolates fell within genogroup E, for which only a single genetic cluster was identified. More striking was that IHNV has evolved more rapidly than VHSV (mean rates of 11 and 7.3 × 10(-4) nucleotide substitutions per site, per year, respectively), indicating that these viruses exhibit fundamentally different evolutionary dynamics. The time to the most recent common ancestor of both IHNV and VHSV was consistent with the first reports of these pathogens in Italy. By combining sequence data with epidemiological information it was possible to identify different patterns of virus spread among trout farms, in which adjacent facilities can be infected by either genetically similar or different viruses, and farms located in different water catchments can be infected by identical strains. Overall, these findings highlight the importance of combining molecular and epidemiological information to identify the determinants of IHN and VHS spread, and to provide data that is central to future surveillance strategies and possibly control.
Keywords: IHNV; VHSV; evolution; molecular epidemiology; phylogeny.
Figures


References
-
- Béarzotti M., Monnier A. F., Vende P., Grosclaude J., de Kinkelin P., Benmansour A. (1995). The glycoprotein of viral hemorrhagic septicemia virus (VHSV): antigenicity and role in virulence. Vet. Res. 26, 413–22. - PubMed
-
- Bovo G., Giorgetti G., Jørgensen P. E. V., Olesen N. J. (1987). Infectious haematopoietic necrosis: first detection in Italy. Bull. Eur. Assoc. Fish Pathol. 7:124.
LinkOut - more resources
Full Text Sources
Other Literature Sources

