A new paradigm of DNA synthesis: three-metal-ion catalysis
- PMID: 27602203
- PMCID: PMC5012070
- DOI: 10.1186/s13578-016-0118-2
A new paradigm of DNA synthesis: three-metal-ion catalysis
Erratum in
-
Erratum to: A new paradigm of DNA synthesis: three-metal-ion catalysis.Cell Biosci. 2017 Jun 20;7:32. doi: 10.1186/s13578-017-0159-1. eCollection 2017. Cell Biosci. 2017. PMID: 28649319 Free PMC article.
Abstract
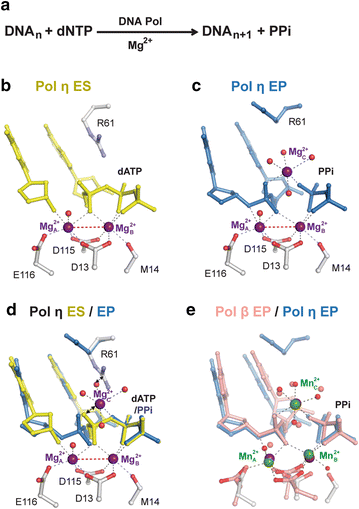
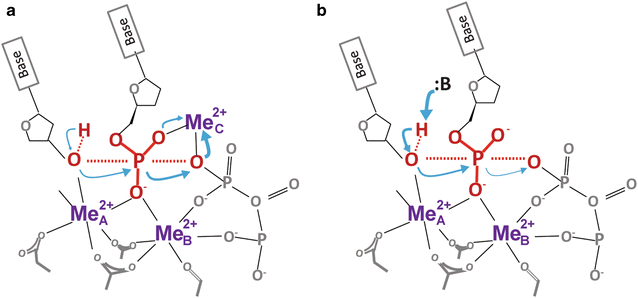
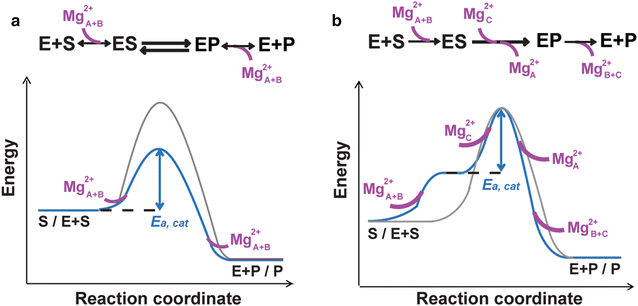
Enzyme catalysis has been studied for over a century. How it actually occurs has not been visualized until recently. By combining in crystallo reaction and X-ray diffraction analysis of reaction intermediates, we have obtained unprecedented atomic details of the DNA synthesis process. Contrary to the established theory that enzyme-substrate complexes and transition states have identical atomic composition and catalysis occurs by the two-metal-ion mechanism, we have discovered that an additional divalent cation has to be captured en route to product formation. Unlike the canonical two metal ions, which are coordinated by DNA polymerases, this third metal ion is free of enzyme coordination. Its location between the α- and β-phosphates of dNTP suggests that the third metal ion may drive the phosphoryltransfer from the leaving group opposite to the 3'-OH nucleophile. Experimental data indicate that binding of the third metal ion may be the rate-limiting step in DNA synthesis and the free energy associated with the metal-ion binding can overcome the activation barrier to the DNA synthesis reaction.
Figures
References
-
- Sumner JB. The isolation and crystallization of the enzyme urease: preliminary paper. JBC. 1926;69:435–441.
-
- Laidler KJ, King MC. Development of transition-state theory. J Phys Chem. 1983;87:2657–2664. doi: 10.1021/j100238a002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

