Guiding Empiric Treatment for Serious Bacterial Infections via Point of Care [Formula: see text]-Lactamase Characterization
- PMID: 27602307
- PMCID: PMC5003167
- DOI: 10.1109/JTEHM.2016.2573305
Guiding Empiric Treatment for Serious Bacterial Infections via Point of Care [Formula: see text]-Lactamase Characterization
Abstract
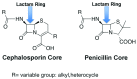
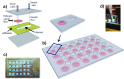
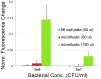
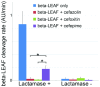
Fever is one of the most common symptoms of illness in infants and represents a clinical challenge due to the potential for serious bacterial infection. As delayed treatment for these infections has been correlated with increased morbidity and mortality, broad-spectrum [Formula: see text]-lactam antibiotics are often prescribed while waiting for microbiological lab results (1-3 days). However, the spread of antibiotic resistance via the [Formula: see text]-lactamase enzyme, which can destroy [Formula: see text]-lactam antibiotics, has confounded this paradigm; empiric antibiotic regimens are increasingly unable to cover all potential bacterial pathogens, leaving some infants effectively untreated until the pathogen is characterized. This can lead to lifelong sequela or death. Here, we introduce a fluorescent, microfluidic assay that can characterize [Formula: see text]-lactamase derived antibiotic susceptibility in 20 min with a sensitivity suitable for direct human specimens. The protocol is extensible, and the antibiotic spectrum investigated can be feasibly adapted for the pathogens of regional relevance. This new assay fills an important need by providing the clinician with hitherto unavailable point of care information for treatment guidance in an inexpensive and simple diagnostic format.
Keywords: Antibiotic resistance; beta-lactamase; microfluidic; point of care.
Figures






Similar articles
-
Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated With Inadequate and Broad-Spectrum Empiric Antibiotic Use.JAMA Netw Open. 2020 Apr 1;3(4):e202899. doi: 10.1001/jamanetworkopen.2020.2899. JAMA Netw Open. 2020. PMID: 32297949 Free PMC article.
-
Pseudomonas aeruginosa chromosomal beta-lactamase in patients with cystic fibrosis and chronic lung infection. Mechanism of antibiotic resistance and target of the humoral immune response.APMIS Suppl. 2003;(116):1-47. APMIS Suppl. 2003. PMID: 14692154 Review.
-
Rapid, low-cost fluorescent assay of β-lactamase-derived antibiotic resistance and related antibiotic susceptibility.J Biomed Opt. 2014;19(10):105007. doi: 10.1117/1.JBO.19.10.105007. J Biomed Opt. 2014. PMID: 25321396 Free PMC article.
-
Microbiological surveillance and antimicrobial stewardship minimise the need for ultrabroad-spectrum combination therapy for treatment of nosocomial infections in a trauma intensive care unit: an audit of an evidence-based empiric antimicrobial policy.S Afr Med J. 2013 Mar 15;103(6):371-6. doi: 10.7196/samj.6459. S Afr Med J. 2013. PMID: 23725954
-
Hospital-acquired respiratory tract infections: clinical experience with beta-lactam/beta-lactamase inhibitors.Int J Clin Pract Suppl. 2002 Mar;(125):19-27; discussion 37-9. Int J Clin Pract Suppl. 2002. PMID: 12014853 Review.
Cited by
-
Photodynamic therapy, priming and optical imaging: Potential co-conspirators in treatment design and optimization - a Thomas Dougherty Award for Excellence in PDT paper.J Porphyr Phthalocyanines. 2020 Nov-Dec;24(11n12):1320-1360. doi: 10.1142/s1088424620300098. J Porphyr Phthalocyanines. 2020. PMID: 37425217 Free PMC article.
-
Collaborative Paradigm of Preventive, Personalized, and Precision Medicine With Point-of-Care Technologies.IEEE J Transl Eng Health Med. 2016 Dec 9;4:2800908. doi: 10.1109/JTEHM.2016.2635126. eCollection 2016. IEEE J Transl Eng Health Med. 2016. PMID: 28560119 Free PMC article.
-
Developing a hybrid antimicrobial resistance surveillance system in India: Needs & challenges.Indian J Med Res. 2019 Feb;149(2):299-302. doi: 10.4103/ijmr.IJMR_2074_17. Indian J Med Res. 2019. PMID: 31219099 Free PMC article.
References
-
- Bachur R. G. and Harper M. B., “Predictive model for serious bacterial infections among infants younger than 3 months of age,” Pediatrics, vol. 108, no. , pp. 311–316, Aug. 2001. - PubMed
-
- Schwartz S., Raveh D., Toker O., Segal G., Godovitch N., and Schlesinger Y., “A week-by-week analysis of the low-risk criteria for serious bacterial infection in febrile neonates,” Arch. Disease Childhood, vol. 94, pp. 287–292, Apr. 2009. - PubMed
-
- ACEP Clinical Policies Committee, “Clinical policy for children younger than three years presenting to the emergency department with fever,” Ann. Emerg. Med., vol. 42, no. 3, pp. 530–545, 2003. - PubMed
-
- Greenhow T. L., Hung Y. Y., and Herz A. M., “Changing epidemiology of bacteremia in infants aged 1 week to 3 months,” Pediatrics, vol. 129, no. 3, pp. e590–e596, Mar. 2012. - PubMed
-
- Greenhow T. L., Hung Y.-Y., Herz A. M., Losada E., and Pantell R. H., “The changing epidemiology of serious bacterial infections in young infants,” Pediatric Infectious Disease J., vol. 33, no. 6, pp. 595–599, Jun. 2014. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

