Dietary supplementation with myo-inositol in women during pregnancy for treating gestational diabetes
- PMID: 27602537
- PMCID: PMC6457692
- DOI: 10.1002/14651858.CD012048.pub2
Dietary supplementation with myo-inositol in women during pregnancy for treating gestational diabetes
Abstract
Background: Gestational diabetes mellitus (GDM) is any degree of glucose intolerance that first presents and is recognised during pregnancy and usually resolves after the birth of the baby. GDM is associated with increased short- and long-term morbidity for the mother and her baby. Treatment usually includes lifestyle modification and/or pharmacological therapy (oral antidiabetic agents or insulin) with the aim to maintain treatment targets for blood glucose concentrations. Finding novel treatment agents which are effective, acceptable and safe for the mother and her baby are important. One such emerging potential intervention is myo-inositol which is an isomer of inositol and occurs endogenously and is found in natural dietary sources such as fruits, vegetables, nuts and cereals.
Objectives: To assess if dietary supplementation with myo-inositol during pregnancy is safe and effective, for the mother and fetus, in treating gestational diabetes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 April 2016), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (7 April 2016), and reference lists of retrieved studies.
Selection criteria: All published and unpublished randomised controlled trials or cluster-randomised controlled trials reporting on the use of myo-inositol compared with placebo, no treatment or another intervention for the treatment of women with gestational diabetes. Quasi-randomised and cross-over studies are not eligible for inclusion. Women with pre-existing diabetes were excluded.
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. For key outcomes (where data were available), we assessed the quality of the evidence using the GRADE approach.
Main results: We included two studies (142 women and infants), both were conducted in women in Italy and compared myo-inositol with a placebo control.None of the maternal primary outcomes pre-specified for this review were reported in the included studies: hypertensive disorders of pregnancy; caesarean section; development of subsequent type 2 diabetes mellitus. No data were reported for the majority of this review's maternal secondary outcomes. We could only perform meta-analysis for two secondary outcomes: fasting oral glucose tolerance test and additional pharmacological treatment. All other results are based on data from single studies. Overall, the risk of bias of the included studies was judged to be unclear due to lack of key methodological information.There was no evidence of a difference between treatment groups in need for additional pharmacotherapy or weight gain during pregnancy, although myo-inositol was associated with a lower body mass index (BMI) change (mean difference (MD) -1.50 kg/m2; 95% confidence interval (CI) -2.35 to -0.65; one trial, n = 73). Myo-inositol was associated with a reduction in the fasting blood glucose concentration at the end of treatment (MD -0.47 mmol/L; 95% CI -0.59 to -0.35; two trials, n = 142 women) compared with the control group. One small trial reported that myo-inositol was associated with a reduction in one-hour post-prandial blood glucose concentration at the end of treatment (MD -0.90 mmol/L; 95% CI -1.73 to -0.07; one trial, n = 73 women) compared with the control group. There was no difference between groups for the two-hour post-prandial blood glucose concentrations between groups (MD -0.70 mmol/L; 95% CI -1.46 to 0.06; one trial, n = 73 women). The one-hour and two-hour blood glucose concentrations show evidence of imprecision associated with wide CIs and small sample size.For the infant, there was no evidence of a difference in the risk for being born large-for-gestational age between the myo-inositol and the control group (risk ratio (RR) 0.36; 95% CI 0.02 to 8.58; one trial, n = 73 infants; low-quality evidence). The evidence was downgraded due to imprecision. This review's other primary outcomes were not reported in the included trials: perinatal mortality (stillbirth and neonatal mortality); mortality of morbidity composite (as defined by the trials); neurosensory disability. Infants in the myo-inositol group were less likely to have neonatal hypoglycaemia compared with the placebo group (RR 0.05; 95% CI 0.00 to 0.85; one study, n = 73 infants; low-quality evidence). There is evidence of imprecision for this outcome with low event rates and small sample size. There was no evidence of a difference between treatment and placebo groups for preterm birth or birthweight. Myo-inositol was associated with a later gestational age at birth compared with the placebo group (MD 2.10 weeks; 95% CI 1.27 to 2.93; one trial, n = 73 infants). No data were reported for any of the other neonatal outcomes for this review.No long-term outcomes were reported for the mother, infant as a child, infant as an adult, or health service outcomes.
Authors' conclusions: There are insufficient data to evaluate the effect of myo-inositol for the treatment of gestational diabetes, with no data to examine the majority of outcomes in this review. There do not appear to be any benefits for the infant associated with exposure to myo-inositol such as reduced risk of being born large-for-gestational age. Although the risk of neonatal hypoglycaemia is reduced for the myo-inositol group, there is evidence of imprecision. Evidence from two studies suggested that myo-inositol was associated with a reduced change in maternal BMI and fasting blood sugar concentration compared with placebo. There is a lack of reporting of the clinically meaningful outcomes pre-specified for this review.Uncertainty of the effectiveness of myo-inositol as a treatment for GDM for key maternal and infant outcomes remains and further high- quality trials with appropriate sample sizes are required to further investigate the role of myo-inositol as a treatment or co-treatment for women with gestational diabetes. Future trials should report on the core outcomes for GDM identified in the methods section of this review. Participants of varying ethnicities and with varying risk factors for GDM should be included in future trials. In addition, further trials of myo-inositol for the treatment of GDM should explore the optimal dose, frequency and timing of supplementation, report on adverse effects and assess the long- term effects of this intervention. Economic analysis or health service use and costs should also be included.
Conflict of interest statement
Tineke J Crawford: none known
Caroline A Crowther: none known
Jane Alsweiler: none known
Julie Brown: none known
Figures
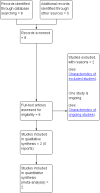
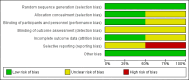
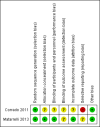











Comment in
-
Dietary supplementation with myo-inositol in women during pregnancy for treating gestational diabetes.Int J Nurs Pract. 2018 Dec;24(6):e12684. doi: 10.1111/ijn.12684. Epub 2018 Jul 22. Int J Nurs Pract. 2018. PMID: 30033626 No abstract available.
References
References to studies included in this review
Corrado 2011 {published data only}
-
- Corrado F, D'Anna R, Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabetic Medicine 2011;28(8):972‐5. - PubMed
-
- NCT00734448. Myo‐inositol administration in gestational diabetes. clinicaltrials.gov/show/NCT00734448 Date first received: 16 April 2008.
Matarrelli 2013 {published data only}
-
- Matarrelli B, Vitacolonna E, D'Angelo M, Pavone G, Celentano C. Early treatment of gestational diabetes and obstetric adverse outcome prevention. Ultrasound in Obstetrics & Gynecology 2011;38(Suppl 1):54.
-
- Matarrelli B, Vitacolonna E, D'angelo M, Pavone G, Mattei PA, Liberati M, et al. Effect of dietary myo‐inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(10):967‐72. - PubMed
-
- NCT01342874. Improved outcomes associated with inositol dietary supplementation in women with gestational diabetes mellitus (inositol). clinicaltrials.gov/show/NCT01342874 Dare first received: 19 April 2011.
References to studies excluded from this review
Malvasi 2014 {published data only}
-
- Malvasi A, Casciaro F, Minervini MM, Kosmas I, Mynbaev OA, Pacella E, et al. Myo‐inositol, D‐chiro‐inositol, folic acid and manganese in second trimester of pregnancy: a preliminary investigation. European Review for Medical and Pharmacological Sciences 2014;18(2):270‐4. - PubMed
Valent 2014 {unpublished data only}
-
- NCT02149992. Glycaemic impact of myo‐inositol in pregnancy. clinicaltrials.gov/show/NCT02149992 Date first received: 25 May 2014.
References to ongoing studies
Vitacolonna 2014 {published data only}
-
- NCT02097069. Inositol stereoisomers to treat gestational diabetes. clinicaltrials.gov/show/NCT02097069 Date first received: 27 February 2014.
Additional references
ACOG 2013
-
- American College of Obstetricians and Gynecologists. Gestational Diabetes Mellitus: Practice Bulletin Number 137. Obstetrics and Gynecology 2013;122(2 Pt 1):406‐16. - PubMed
ADA 2008
-
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2008;31(Suppl 1):S55‐S60. - PubMed
Alberti 1998
-
- Alberti K, Zimmet P. Definition, diagnosis and classification of diabetes mellitus. Part 1: Diagnosis and classification of diabetes mellitus: World Health Organization Report. Diabetic Medicine 1998;15(7):539‐53. - PubMed
Alwan 2009
Boney 2005
-
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115(3):e290‐6. - PubMed
Carlomagno 2011
-
- Carlomagno G, Unfer V, Buffo S, D'Ambrosio F. Myo‐inositol in the treatment of premenstrual dysphoric disorder. Human Psychopharmacology: Clinical and Experimental 2011;26(7):526‐30. - PubMed
Catalano 2003
-
- Catalano P, Thomas A, Huston‐Presley L, Amini S. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. American Journal of Obstetrics and Gynecology 2003;189(6):1698‐704. - PubMed
CDA 2008
-
- Canadian Diabetes Association. Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian Journal of Diabetes 2008;32(Suppl 1):S1‐S201. - PubMed
Clements 1980
-
- Clements RS, Darnell B. Myo‐inositol content of common foods: development of a high myo‐inositol diet. American Journal of Clinical Nutrition 1980;33:1954‐67. - PubMed
Cohen 2006
-
- Cohen P. The twentieth century struggle to decipher insulin signalling. Nature Reviews. Molecular Cell Biology 2006;7:867‐73. - PubMed
Crawford 2015
Crowther 2005
-
- Crowther C, Hiller J, Moss J, McPhee A, Jeffries W, Robinson J. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352:2477‐86. - PubMed
Croze 2013
-
- Croze ML, Soulage CO. Potential role and therapeutic interests of myo‐inositol in metabolic diseases. Biochimie 2013;95(10):1811‐27. - PubMed
D'Anna 2012
-
- D'Anna R, Bendetto V, Rizzo P, Raffone E, Interdanto ML, Corado F, et al. Myo‐inositol may prevent gestational diabetes in PCOS women. Gynecological Endocrinology 2012;28(6):440‐2. - PubMed
Ferrara 2007
-
- Ferrara A. Increasing prevalence of gestational diabetes mellitus. Diabetes Care 2007;30(Suppl 2):S141‐S146. - PubMed
Genazzani 2008
-
- Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo‐inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovarian syndrome. Gynecological Endocrinology 2008;24:139‐44. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IADPSG 2010
Kim 2002
-
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862‐8. - PubMed
Landon 2009
Larner 2010
Lubin 2016
McCance 2011
-
- McCance DR. Pregnancy and diabetes. Best Practice & Research Clinical Endocrinology & Metabolism 2011;25:945‐58. - PubMed
McCurdy 2010
-
- McCurdy C, Friedman J. Mechanisms underlying insulin resistance in human pregnancy and gestational diabetes mellitus. In: Kim K, Ferrara A editor(s). Gestational Diabetes During and After Pregnancy. London: Springer, 2010:125‐38.
Metzger 1998
-
- Metzger BE, Coustan DR. Proceedings of the Fourth International Workshop‐Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21 (Suppl. 2):B1‐B167. - PubMed
Minozzi 2008
-
- Minozzi M, D'Andrea G, Unfer V. Treatment of hirsutism with myo‐inositol: a prospective clinical study. Reproductive Biomedicine Online 2008;17(4):579‐82. - PubMed
Nankervis 2013
-
- Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C, et al. for the Australasian Diabetes in Pregnancy Society (ADIPS). Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia. URL: http://adips.org/downloads/ADIPSConsensusGuidelinesGDM‐03.05.13VersionAC... (accessed 03 July 2015).
NCT00734448
-
- NCT00734448. Myo‐inositol administration in gestational diabetes. clinicaltrials.gov/show/NCT00734448 Date first received: 16 April 2008.
New Zealand Ministry of Health 2014
-
- New Zealand Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline. Wellington, New Zealand: Ministry of Health, 2014.
NICE 2015
-
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre‐conception to the Postnatal Period. Manchester: NICE, 2015.
Nordio 2013
Papaleo 2007
-
- Papaleo E, Unfer V, Baillargeon JP, Santis L, Fusi F, Brigante C, et al. Myoinositol in patients with polycystic ovarian syndrome: a novel method for ovulation induction. Gynecological Endocrinology 2007;23:700‐3. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saltiel 1990
-
- Saltiel AR. Second messengers of insulin action. Diabetes Care 1990;13(3):244‐56. - PubMed
The HAPO Study Cooperative Research Group 2008
-
- The HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358(19):1991‐2002. - PubMed
Unfer 2011
-
- Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo‐inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecological Endocrinology 2011;27:857‐61. - PubMed
Wang 2013
-
- Wang Z, Kanguru L, Hussein J, Fitzmaurice A, Ritchie K. Incidence of adverse outcomes associated with gestational diabetes mellitus in low‐ and middle‐income countries. International Journal of Gynecology and Obstetrics 2013;121:14‐9. - PubMed
Wendland 2012
-
- Wendland E, Torloni M, Falavigna M, Trujilo J, Dose MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes: a systematic review of the World Health Organization (WHO) and the International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy and Childbirth 2012;12:23. - PMC - PubMed
References to other published versions of this review
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

