The Charge Properties of Phospholipid Nanodiscs
- PMID: 27602726
- PMCID: PMC5018125
- DOI: 10.1016/j.bpj.2016.06.041
The Charge Properties of Phospholipid Nanodiscs
Abstract
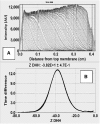
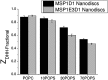
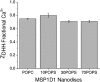
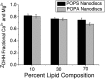
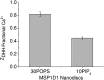
Phospholipids (PLs) are a major, diverse constituent of cell membranes. PL diversity arises from the nature of the fatty acid chains, as well as the headgroup structure. The headgroup charge is thought to contribute to both the strength and specificity of protein-membrane interactions. Because it has been difficult to measure membrane charge, ascertaining the role charge plays in these interactions has been challenging. Presented here are charge measurements on lipid Nanodiscs at 20°C in 100 mM NaCl, 50 mM Tris, at pH 7.4. Values are also reported for measurements made in the presence of Ca(2+) and Mg(2+) as a function of NaCl concentration, pH, and temperature, and in solvents containing other types of cations and anions. Measurements were made for neutral (phosphatidylcholine and phosphatidylethanolamine) and anionic (phosphatidylserine, phosphatidic acid, cardiolipin, and phosphatidylinositol 4,5-bisphosphate (PIP2)) PLs containing palmitoyl-oleoyl and dimyristoyl fatty acid chains. In addition, charge measurements were made on Nanodiscs containing an Escherichia coli lipid extract. The data collected reveal that 1) POPE is anionic and not neutral at pH 7.4; 2) high-anionic-content Nanodiscs exhibit polyelectrolyte behavior; 3) 3 mM Ca(2+) neutralizes a constant fraction of the charge, but not a constant amount of charge, for POPS and POPC Nanodiscs; 4) in contrast to some previous work, POPC only interacts weakly with Ca(2+); 5) divalent cations interact with lipids in a lipid- and ion-specific manner for POPA and PIP2 lipids; and 6) the monovalent anion type has little influence on the lipid charge. These results should help eliminate inconsistencies among data obtained using different techniques, membrane systems, and experimental conditions, and they provide foundational data for developing an accurate view of membranes and membrane-protein interactions.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Edsall J.T., Wyman J. Academic Press; New York: 1958. Biophysical Chemistry, Vol. I: Thermodynamics, Electrostatics, and the Biological Significance of the Properties of Matter.
-
- O’Brien R.W., White L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc., Faraday Trans. II. 1978;74:1607–1626.
-
- Scatchard G., Black E.S. The effect of salts on the isoionic and isoelectric points of proteins. J. Phys. Colloid Chem. 1949;53:88–99. - PubMed
-
- Hayes, D. B. 1993. Equilibrium electrophoresis: results from the second prototype. PhD thesis. University of New Hampshire, Durham, NH.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

