Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells
- PMID: 27602757
- PMCID: PMC5323208
- DOI: 10.18632/oncotarget.11786
Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells
Abstract
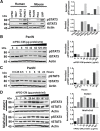
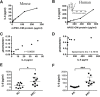
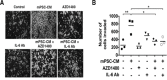
Pancreatic ductal adenocarcinoma (PDAC) is a dynamic tumor supported by several stromal elements such as pancreatic stellate cells (PSC). Significant crosstalk exists between PSCs and tumor cells to stimulate oncogenic signaling and malignant progression of PDAC. However, how PSCs activate intercellular signaling in PDAC cells remains to be elucidated. We have previously shown that activated signal transducer and activator of transcription 3 (STAT3) signaling is a key component in the progression of pancreatic neoplasia. We hypothesize that PSC secreted IL-6 activates STAT3 signaling to promote PanIN progression to PDAC. Human PDAC and mouse PanIN cells were treated with PSC-conditioned media (PSC-CM), and phospho- and total-STAT3 levels by immunoblot analysis were determined. IL-6 was quantified in PSC-CM and cell invasion and colony formation assays were performed in the presence or absence of a neutralizing IL-6 antibody and the JAK/STAT3 inhibitor AZD1480. Serum from Ptf1aCre/+;LSL-KrasG12D/+;Tgfbr2flox/flox (PKT) and LSL-KrasG12D/+; Trp53R172H/+; Pdx1Cre/+ (KPC) mice demonstrated increased levels of IL-6 compared to serum from non-PDAC bearing KC and PK mice. PSC secreted IL-6 activated STAT3 signaling in noninvasive, precursor PanIN cells as well as PDAC cells, resulting in enhanced cell invasion and colony formation in both cell types. There was a significant positive linear correlation between IL-6 concentration and the ratio of phosphorylated STAT3/total STAT3. IL-6 neutralization or STAT3 inhibition attenuated PSC-CM induced activation of STAT3 signaling and tumorigenicity. These data provide evidence that PSCs are directly involved in promoting the progression of PanINs towards invasive carcinoma. This study demonstrates a novel role of PSC secreted IL-6 in transitioning noninvasive pancreatic precursor cells into invasive PDAC through the activation of STAT3 signaling.
Keywords: IL-6; PanIN; STAT3; pancreatic cancer; pancreatic stellate cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Paracrine IL-6 signaling mediates the effects of pancreatic stellate cells on epithelial-mesenchymal transition via Stat3/Nrf2 pathway in pancreatic cancer cells.Biochim Biophys Acta Gen Subj. 2017 Feb;1861(2):296-306. doi: 10.1016/j.bbagen.2016.10.006. Epub 2016 Oct 14. Biochim Biophys Acta Gen Subj. 2017. PMID: 27750041
-
Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article.
-
Galectin-3 Mediates Tumor Cell-Stroma Interactions by Activating Pancreatic Stellate Cells to Produce Cytokines via Integrin Signaling.Gastroenterology. 2018 Apr;154(5):1524-1537.e6. doi: 10.1053/j.gastro.2017.12.014. Epub 2017 Dec 21. Gastroenterology. 2018. PMID: 29274868
-
Persistent activation of pancreatic stellate cells creates a microenvironment favorable for the malignant behavior of pancreatic ductal adenocarcinoma.Int J Cancer. 2013 Mar 1;132(5):993-1003. doi: 10.1002/ijc.27715. Epub 2012 Oct 5. Int J Cancer. 2013. PMID: 22777597 Review.
-
Pancreatic stellate cell: Pandora's box for pancreatic disease biology.World J Gastroenterol. 2017 Jan 21;23(3):382-405. doi: 10.3748/wjg.v23.i3.382. World J Gastroenterol. 2017. PMID: 28210075 Free PMC article. Review.
Cited by
-
Single-cell RNA sequencing to explore cancer-associated fibroblasts heterogeneity: "Single" vision for "heterogeneous" environment.Cell Prolif. 2024 May;57(5):e13592. doi: 10.1111/cpr.13592. Epub 2023 Dec 29. Cell Prolif. 2024. PMID: 38158643 Free PMC article. Review.
-
Pancreatic cancer stroma: an update on therapeutic targeting strategies.Nat Rev Gastroenterol Hepatol. 2020 Aug;17(8):487-505. doi: 10.1038/s41575-020-0300-1. Epub 2020 May 11. Nat Rev Gastroenterol Hepatol. 2020. PMID: 32393771 Free PMC article. Review.
-
CAR-Macrophage Cell Therapy: A New Era of Hope for Pancreatic Cancer.Clin Cancer Res. 2025 Aug 4:10.1158/1078-0432.CCR-25-1201. doi: 10.1158/1078-0432.CCR-25-1201. Online ahead of print. Clin Cancer Res. 2025. PMID: 40757874 Free PMC article.
-
Identification of Functional Heterogeneity of Carcinoma-Associated Fibroblasts with Distinct IL6-Mediated Therapy Resistance in Pancreatic Cancer.Cancer Discov. 2022 Jun 2;12(6):1580-1597. doi: 10.1158/2159-8290.CD-20-1484. Cancer Discov. 2022. PMID: 35348629 Free PMC article.
-
Beyond just a tight fortress: contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer.Signal Transduct Target Ther. 2020 Oct 30;5(1):249. doi: 10.1038/s41392-020-00341-1. Signal Transduct Target Ther. 2020. PMID: 33122631 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

