ctDNA dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy
- PMID: 27602761
- PMCID: PMC5323211
- DOI: 10.18632/oncotarget.11791
ctDNA dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy
Abstract
Background: Most studies utilizing circulating tumor DNA (ctDNA) to monitor disease interrogated only one or a few genes and failed to develop workable criteria to inform clinical practice. We evaluated the feasibility of detecting resistance to anti-HER2 therapy by serial gene-panel ctDNA sequencing.
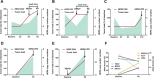
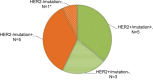
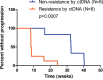
Results: Primary therapeutic resistance was identified in 6 out of 14 patients with events of progressive disease. For this subset comparison of pre- and post-treatment ctDNA assay results revealed that HER2 amplification concurred with disease progression (4/6, 66.7%). Mutations in TP53 (3/6, 50.0%) and genes implicated in the PI3K/mTOR pathway (3/6, 50.0%) were also dominant markers of resistance. Together, resistance to HER2 blockade should be indicated during treatment if any of the following situations applies: 1) recurrence or persistence of HER2 amplification in the blood; 2) emergence or ≥20% increase in the fraction of mutations in any of these resistance-related genes including TP53/PIK3CA/MTOR/PTEN. Compared with CT scans, dynamic ctDNA profiling utilizing pre-defined criteria was sensitive in identifying drug resistance (sensitivity 85.7%, specificity 55.0%), with a concordance rate up to 82.1%. Besides, the ctDNA criteria had a discriminating role in the prognosis of HER2-positive metastatic breast cancer.
Methods: 52 plasma samples were prospectively collected from 18 patients with HER2-positive metastatic breast cancer who were treated with an oral anti-HER1/HER2 tyrosine kinase inhibitor (ClinicalTrials.gov NCT01937689). ctDNA was assayed by gene-panel target-capture next-generation sequencing.
Conclusions: Longitudinal gene-panel ctDNA sequencing could be exploited to determine resistance and guide the precise administration of anti-HER2 targeted therapy in the metastatic setting.
Keywords: anti-HER2 therapy; circulating tumor DNA; dynamics; metastatic breast cancer; progression.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures

Similar articles
-
Assessing tumor heterogeneity using ctDNA to predict and monitor therapeutic response in metastatic breast cancer.Int J Cancer. 2020 Mar 1;146(5):1359-1368. doi: 10.1002/ijc.32536. Epub 2019 Jul 4. Int J Cancer. 2020. PMID: 31241775 Clinical Trial.
-
Monitoring treatment efficacy and resistance in breast cancer patients via circulating tumor DNA genomic profiling.Mol Genet Genomic Med. 2020 Feb;8(2):e1079. doi: 10.1002/mgg3.1079. Epub 2019 Dec 23. Mol Genet Genomic Med. 2020. PMID: 31867841 Free PMC article.
-
Identifying Circulating Tumor DNA Mutation Profiles in Metastatic Breast Cancer Patients with Multiline Resistance.EBioMedicine. 2018 Jun;32:111-118. doi: 10.1016/j.ebiom.2018.05.015. Epub 2018 May 26. EBioMedicine. 2018. PMID: 29807833 Free PMC article.
-
Review ctDNA and Breast Cancer.Recent Results Cancer Res. 2020;215:231-252. doi: 10.1007/978-3-030-26439-0_12. Recent Results Cancer Res. 2020. PMID: 31605232 Review.
-
Association of Circulating Tumor DNA With Disease-Free Survival in Breast Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Nov 2;3(11):e2026921. doi: 10.1001/jamanetworkopen.2020.26921. JAMA Netw Open. 2020. PMID: 33211112 Free PMC article.
Cited by
-
Circulating tumor DNA analysis in the era of precision oncology.Oncotarget. 2020 Jan 14;11(2):188-211. doi: 10.18632/oncotarget.27418. eCollection 2020 Jan 14. Oncotarget. 2020. PMID: 32010431 Free PMC article. Review.
-
Overexpression of Stat3 increases circulating cfDNA in breast cancer.Breast Cancer Res Treat. 2021 May;187(1):69-80. doi: 10.1007/s10549-021-06142-6. Epub 2021 Feb 25. Breast Cancer Res Treat. 2021. PMID: 33630196
-
Real-time assessment of HER2 status in circulating tumor cells of breast cancer patients: Methods of detection and clinical implications.J Liq Biopsy. 2023 Oct 8;2:100117. doi: 10.1016/j.jlb.2023.100117. eCollection 2023 Dec. J Liq Biopsy. 2023. PMID: 40028485 Free PMC article. Review.
-
Efficacy of Pyrotinib in a Heavily Pretreated Patient with Lung Adenocarcinoma Harboring HER2 Amplification and Exon 20 Insertions: A Case Report.Onco Targets Ther. 2020 Oct 2;13:9849-9856. doi: 10.2147/OTT.S271999. eCollection 2020. Onco Targets Ther. 2020. PMID: 33061462 Free PMC article.
-
A Phenomic Perspective on Factors Influencing Breast Cancer Treatment: Integrating Aging and Lifestyle in Blood and Tissue Biomarker Profiling.Front Immunol. 2021 Feb 1;11:616188. doi: 10.3389/fimmu.2020.616188. eCollection 2020. Front Immunol. 2021. PMID: 33597950 Free PMC article. Review.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. - PubMed
-
- Seshadri R, Firgaira FA, Horsfall DJ, McCaul K, Setlur V, Kitchen P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol. 1993;11:1936–1942. - PubMed
-
- Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, Danyluk J, Godolphin W, Sliwkowski M, Akita R. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. - PubMed
-
- Ravdin PM, Chamness GC. The c-erbB-2 proto-oncogene as a prognostic and predictive marker in breast cancer: a paradigm for the development of other macromolecular markers--a review. Gene. 1995;159:19–27. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

