Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR
- PMID: 27602954
- PMCID: PMC5348338
- DOI: 10.18632/oncotarget.11825
Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR
Abstract
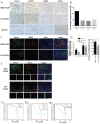
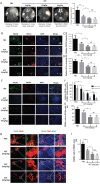
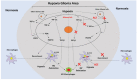
Tumor-associated macrophages (TAMs) are enriched in gliomas and help create a tumor-immunosuppressive microenvironment. A distinct M2-skewed type of macrophages makes up the majority of glioma TAMs, and these cells exhibit pro-tumor functions. Gliomas contain large hypoxic areas, and the presence of a correlation between the density of M2-polarized TAMs and hypoxic areas suggests that hypoxia plays a supportive role during TAM recruitment and induction. Here, we investigated the effects of hypoxia on human macrophage recruitment and M2 polarization. We also investigated the influence of the HIF inhibitor acriflavine (ACF) on M2 TAM infiltration and tumor progression in vivo. We found that hypoxia increased periostin (POSTN) expression in glioma cells and promoted the recruitment of macrophages. Hypoxia-inducible POSTN expression was increased by TGF-α via the RTK/PI3K pathway, and this effect was blocked by treating hypoxic cells with ACF. We also demonstrated that both a hypoxic environment and hypoxia-treated glioma cell supernatants were capable of polarizing macrophages toward a M2 phenotype. ACF partially reversed the M2 polarization of macrophages by inhibiting the upregulation of M-CSFR in macrophages and TGF-β in glioma cells under hypoxic conditions. Administering ACF also ablated tumor progression in vivo. Our findings reveal a mechanism that underlies hypoxia-induced TAM enrichment and M2 polarization and suggest that pharmacologically inhibiting HIFs may reduce M2-polarized TAM infiltration and glioma progression.
Keywords: M2 macrophage; acriflavine; glioma; hypoxia; tumor-associated macrophage.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures





References
-
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502–14. - PubMed
-
- Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61:1100–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

