A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study)
- PMID: 27603005
- PMCID: PMC5600677
- DOI: 10.1002/cncr.30286
A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study)
Abstract
Background: The current single-arm, open-label trial was designed to evaluate the activity of apitolisib (GDC-0980), a dual phosphoinositide 3-kinase/mammalian target of rapamycin (PI3K/mTOR) inhibitor, in patients with advanced endometrial cancer (EC).
Methods: Patients with recurrent or persistent EC who were treated with 1 to 2 prior lines of chemotherapy but no prior PI3K/mTOR inhibitor received oral apitolisib at a dose of 40 mg daily during 28-day cycles until disease progression or intolerable toxicity occurred. Patients with type I/II diabetes who required insulin were excluded. The primary endpoints were progression-free survival (PFS) at 6 months and objective response rate.
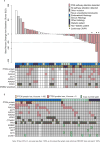
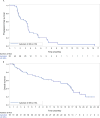
Results: A total of 56 women were enrolled, including 13 (23%) with well-controlled diabetes. Reasons for discontinuation were disease progression (24 patients; 43%), adverse events (13 patients; 23%), and withdrawal by subject (12 patients; 21%). Grade 3/4 apitolisib-related adverse events were hyperglycemia (46%), rash (30%), colitis (5%), and pneumonitis (4%) (toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events [version 4.0]). The PFS rate at 6 months was 20% (Kaplan-Meier estimate; 95% confidence interval [95% CI], 7%-33%). The objective response rate was 6% (confirmed). The median PFS was 3.5 months (95% CI, 2.7-3.7 months) and the median overall survival was 15.7 months (95% CI, 9.2-17.0 months). Nineteen patients discontinued the study before the first tumor assessment. Dose reductions were required for 4 diabetic (31%) and 18 nondiabetic (42%) patients. Comprehensive molecular profiling of 46 evaluable archival tumor samples demonstrated that 57% of patients had at least 1 alteration in phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), phosphatase and tensin homolog (PTEN), or AKT1. All 3 patients with a confirmed response had at least 1 alteration in a PI3K pathway gene.
Conclusions: The antitumor activity noted with the use of a dose of 40 mg of apitolisib daily was limited by tolerability, especially in diabetic patients. Patients with PI3K pathway mutations may have derived enhanced benefit from apitolisib. Cancer 2016;122:3519-28. © 2016 American Cancer Society.
Keywords: GDC-0980; MAGGIE; apitolisib; endometrial cancer.
© 2016 American Cancer Society.
Conflict of interest statement
The following authors have no conflicts of interest to declare: V. Makker, F.O. Recio, and L. Ma. J.O. Lauchle, H. Parmar, H.N. Gilbert, J.A. Ware, R. Zhu, S. Lu, L.Y. Huw, Y. Wang, H. Koeppen, J.M. Spoerke, and M.R. Lackner are employees of Genentech, Inc., South San Francisco, CA, and stockholders of Roche.
The following authors have disclosures: U. Matulonis has served on the advisory board at Genentech and at Astrazeneca; C. Aghajanian has served on the advisory board at AstraZeneca and received travel expenses from Abbvie.
Figures




References
-
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. - PubMed
-
- Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. - PubMed
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

