Celecoxib and sulfasalazine had negative association with coronary artery diseases in patients with ankylosing spondylitis: A nation-wide, population-based case-control study
- PMID: 27603385
- PMCID: PMC5023908
- DOI: 10.1097/MD.0000000000004792
Celecoxib and sulfasalazine had negative association with coronary artery diseases in patients with ankylosing spondylitis: A nation-wide, population-based case-control study
Abstract
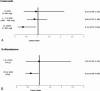
The aim of the study is to assess the effects of celecoxib and sulfasalazine on the risk of coronary artery disease (CAD) in patients with ankylosing spondylitis (AS).Using the claims data of Taiwan National Health Insurance (NHI) database, a nationally representative data that contain the medical records of 23 million Taiwan residents, we randomly selected 1 million cohort from the database, and then we enrolled only patients who were newly diagnosed with AS (n = 4829) between year 2001 and 2010, excluding patients who had CAD (ICD-9- CM codes: 410-414) before the diagnosis of AS (n = 4112). According to propensity score matched 1:2 on age, gender, AS duration, Charlson comorbidity index, hypertension, and hyperlipidemia, 236 and 472 patients were included in the case (AS with CAD) and control (AS without CAD) groups, respectively. We used the WHO defined daily dose (DDD) as a tool to assess the dosage of sulfasalazine and celecoxib exposure. Conditional logistic regression was used to estimate the crude and adjusted odds ratios (ORs) and 95% confidence interval (CI) for the risk of CAD associated with use of sulfasalazine and celecoxib.Among 4112 AS patients, 8.4% (346/4112) developed CAD. CAD in AS patients were positively associated with age of 35 to 65, Charlson comorbidities index (CCI), hypertension, and hyperlipidemia. There was no gender difference between case and control groups. After adjustment for age, gender, CCI, hypertension, and hyperlipidemia, sulfasalazine users with an average daily dose ≥ 0.5 DDD (0.5 gm/day) had negative association with CAD events as compared to sulfasalazine nonusers (OR 0.63; 95% CI, 0.40-0.99, P < 0.05). NSAIDs, including celecoxib, etoricoxib, but no naproxen and diclofenac were negatively associated with CAD. Celecoxib users, with an average daily dose > 1.5 DDD, were negatively associated with CAD events, compared to celecoxib nonusers (OR 0.34; 95% CI, 0.13-0.89; P < 0.05).In this 10-year population-based case-control study, 8.4% of AS patients developed CAD. Sulfasalazine usage at an average dose of ≥ 0.5 gm/day demonstrated negative association with CAD events in patients with AS.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Sulfasalazine might reduce risk of cardiovascular diseases in patients with ankylosing spondylitis: A nationwide population-based retrospective cohort study.Int J Rheum Dis. 2017 Mar;20(3):363-370. doi: 10.1111/1756-185X.12986. Epub 2016 Dec 10. Int J Rheum Dis. 2017. PMID: 27943609
-
Hydroxychloroquine may be associated with reduced risk of coronary artery diseases in patients with rheumatoid arthritis: A nationwide population-based cohort study.Int J Clin Pract. 2018 May;72(5):e13095. doi: 10.1111/ijcp.13095. Epub 2018 Apr 24. Int J Clin Pract. 2018. PMID: 29691971
-
Cost effectiveness of etoricoxib versus celecoxib and non-selective NSAIDS in the treatment of ankylosing spondylitis.Pharmacoeconomics. 2010;28(4):323-44. doi: 10.2165/11314690-000000000-00000. Pharmacoeconomics. 2010. PMID: 20222755
-
[New and old therapeutic options in ankylosing spondylitis--is there an indication for sulfasalazine?].Z Rheumatol. 2002 Apr;61(2):151-8. doi: 10.1007/s003930200023. Z Rheumatol. 2002. PMID: 12056292 Review. German. No abstract available.
-
[Disease-modifying anti-rheumatic drugs for treatment of ankylosing spondylitis].Ugeskr Laeger. 2009 Aug 10;171(33):2268-72. Ugeskr Laeger. 2009. PMID: 19732504 Review. Danish.
Cited by
-
High inflammatory burden predicts cardiovascular events in patients with axial spondyloarthritis: a long-term follow-up study.Ther Adv Musculoskelet Dis. 2022 Sep 8;14:1759720X221122401. doi: 10.1177/1759720X221122401. eCollection 2022. Ther Adv Musculoskelet Dis. 2022. PMID: 36105413 Free PMC article.
-
The effect of anti-rheumatic medications for coronary artery diseases risk in patients with rheumatoid arthritis might be changed over time: A nationwide population-based cohort study.PLoS One. 2017 Jun 28;12(6):e0179081. doi: 10.1371/journal.pone.0179081. eCollection 2017. PLoS One. 2017. PMID: 28658301 Free PMC article.
-
Association of ankylosing spondylitis with cardiovascular disease: a bidirectional two-sample mendelian randomization study.Front Genet. 2024 Jun 26;15:1260247. doi: 10.3389/fgene.2024.1260247. eCollection 2024. Front Genet. 2024. PMID: 38988836 Free PMC article.
-
Should axial spondyloarthritis without radiographic changes be treated with anti-TNF agents?Rheumatol Int. 2017 Mar;37(3):327-336. doi: 10.1007/s00296-016-3635-8. Epub 2016 Dec 29. Rheumatol Int. 2017. PMID: 28035438 Review.
-
Celecoxib exerts protective effects in the vascular endothelium via COX-2-independent activation of AMPK-CREB-Nrf2 signalling.Sci Rep. 2018 Apr 19;8(1):6271. doi: 10.1038/s41598-018-24548-z. Sci Rep. 2018. PMID: 29674687 Free PMC article.
References
-
- Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007; 369:1379–1390. - PubMed
-
- Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med 1999; 340:1888–1899. - PubMed
-
- Hennekens CH, Borzak S. Cyclooxygenase-2 inhibitors and most traditional nonsteroidal anti-inflammatory drugs cause similar moderately increased risks of cardiovascular disease. J Cardiovasc Pharmacol Ther 2008; 13:41–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

