Cardiothoracic manifestations of primary histiocytoses
- PMID: 27603510
- PMCID: PMC5604911
- DOI: 10.1259/bjr.20160347
Cardiothoracic manifestations of primary histiocytoses
Abstract
The objectives of this article were: (1) to review common and rare manifestations of systemic and pulmonary Langerhans cell histiocytosis, Rosai-Dorfman disease, Erdheim-Chester disease and juvenile xanthogranuloma; (2) to provide the reader with important pathologic, epidemiologic and clinical features of these diseases. The histiocytoses are a diverse group of diseases which typically manifest with multiorgan involvement. Understanding the pathologic, epidemiologic and clinical features of these entities can help the radiologist suggest an accurate diagnosis of histiocytosis when typical imaging features are encountered.
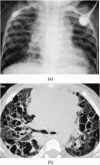
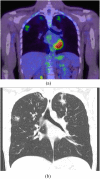
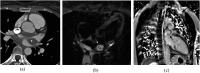
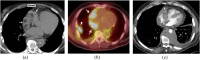
Figures






References
-
- Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, et al. . Contemporary classification of histiocytic disorders. The WHO committee on histiocytic/reticulum cell proliferations. Reclassification working group of the histiocyte society. Med Pediatr Oncol 1997; 29: 157–66. doi: https://doi.org/10.1002/(SICI)1096-911X(199709)29:3<157::AID-MPO1>3.0.CO... - DOI - PubMed
-
- Jaffe R. The histiocytoses. Clin Lab Med 1999; 19: 135–55. - PubMed
-
- Katz SI, Tamaki K, Sachs DH. Epidermal langerhans cells are derived from cells originating in bone marrow. Nature 1979; 282: 324–6. doi: https://doi.org/10.1038/282324a0 - DOI - PubMed
-
- Lieberman PH, Jones CR, Steinman RM, Erlandson RA, Smith J, Gee T, et al. . Langerhans cell (eosinophilic) granulomatosis. A clinicopathologic study encompassing 50 years. Am J Surg Pathol 1996; 20: 519–52. doi: https://doi.org/10.1097/00000478-199605000-00001 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

