Pharmacological Targeting the REV-ERBs in Sleep/Wake Regulation
- PMID: 27603791
- PMCID: PMC5014418
- DOI: 10.1371/journal.pone.0162452
Pharmacological Targeting the REV-ERBs in Sleep/Wake Regulation
Abstract
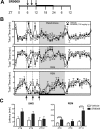
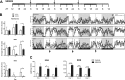
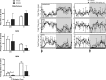
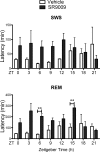
The circadian clock maintains appropriate timing for a wide range of behaviors and physiological processes. Circadian behaviors such as sleep and wakefulness are intrinsically dependent on the precise oscillation of the endogenous molecular machinery that regulates the circadian clock. The identical core clock machinery regulates myriad endocrine and metabolic functions providing a link between sleep and metabolic health. The REV-ERBs (REV-ERBα and REV-ERBβ) are nuclear receptors that are key regulators of the molecular clock and have been successfully targeted using small molecule ligands. Recent studies in mice suggest that REV-ERB-specific synthetic agonists modulate metabolic activity as well as alter sleep architecture, inducing wakefulness during the light period. Therefore, these small molecules represent unique tools to extensively study REV-ERB regulation of sleep and wakefulness. In these studies, our aim was to further investigate the therapeutic potential of targeting the REV-ERBs for regulation of sleep by characterizing efficacy, and optimal dosing time of the REV-ERB agonist SR9009 using electroencephalographic (EEG) recordings. Applying different experimental paradigms in mice, our studies establish that SR9009 does not lose efficacy when administered more than once a day, nor does tolerance develop when administered once a day over a three-day dosing regimen. Moreover, through use of a time response paradigm, we determined that although there is an optimal time for administration of SR9009 in terms of maximal efficacy, there is a 12-hour window in which SR9009 elicited a response. Our studies indicate that the REV-ERBs are potential therapeutic targets for treating sleep problems as those encountered as a consequence of shift work or jet lag.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
REV-ERBβ is required to maintain normal wakefulness and the wake-inducing effect of dual REV-ERB agonist SR9009.Biochem Pharmacol. 2018 Apr;150:1-8. doi: 10.1016/j.bcp.2018.01.009. Epub 2018 Jan 23. Biochem Pharmacol. 2018. PMID: 29355503 Free PMC article.
-
The Putatively Specific Synthetic REV-ERB Agonist SR9009 Inhibits IgE- and IL-33-Mediated Mast Cell Activation Independently of the Circadian Clock.Int J Mol Sci. 2019 Dec 14;20(24):6320. doi: 10.3390/ijms20246320. Int J Mol Sci. 2019. PMID: 31847374 Free PMC article.
-
Pharmacological and Genetic Modulation of REV-ERB Activity and Expression Affects Orexigenic Gene Expression.PLoS One. 2016 Mar 10;11(3):e0151014. doi: 10.1371/journal.pone.0151014. eCollection 2016. PLoS One. 2016. PMID: 26963516 Free PMC article.
-
A role for rev-erbα ligands in regulation of adipogenesis.Curr Pharm Des. 2011;17(4):320-4. doi: 10.2174/138161211795164211. Curr Pharm Des. 2011. PMID: 21375499 Review.
-
The REV-ERB Nuclear Receptors: Timekeepers for the Core Clock Period and Metabolism.Endocrinology. 2023 Apr 17;164(6):bqad069. doi: 10.1210/endocr/bqad069. Endocrinology. 2023. PMID: 37149727 Free PMC article. Review.
Cited by
-
Recent advances in modulators of circadian rhythms: an update and perspective.J Enzyme Inhib Med Chem. 2020 Dec;35(1):1267-1286. doi: 10.1080/14756366.2020.1772249. J Enzyme Inhib Med Chem. 2020. PMID: 32506972 Free PMC article. Review.
-
REV-ERBβ is required to maintain normal wakefulness and the wake-inducing effect of dual REV-ERB agonist SR9009.Biochem Pharmacol. 2018 Apr;150:1-8. doi: 10.1016/j.bcp.2018.01.009. Epub 2018 Jan 23. Biochem Pharmacol. 2018. PMID: 29355503 Free PMC article.
-
Chronic low-dose REV-ERBs agonist SR9009 mitigates constant light-induced weight gain and insulin resistance via adipogenesis modulation.Biomed J. 2025 Jun;48(3):100830. doi: 10.1016/j.bj.2025.100830. Epub 2025 Jan 10. Biomed J. 2025. PMID: 39800061 Free PMC article.
-
Current Perspective on the Role of the Circadian Clock and Extracellular Matrix in Chronic Lung Diseases.Int J Environ Res Public Health. 2023 Jan 30;20(3):2455. doi: 10.3390/ijerph20032455. Int J Environ Res Public Health. 2023. PMID: 36767821 Free PMC article. Review.
-
Circadian Clock in Muscle Disease Etiology and Therapeutic Potential for Duchenne Muscular Dystrophy.Int J Mol Sci. 2024 Apr 27;25(9):4767. doi: 10.3390/ijms25094767. Int J Mol Sci. 2024. PMID: 38731986 Free PMC article. Review.
References
-
- Milagro FI, Gomez-Abellan P, Campion J, Martinez JA, Ordovas JM, Garaulet M. CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiology international. 2012;29(9):1180–94. 10.3109/07420528.2012.719967 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

