Inhomogeneity Based Characterization of Distribution Patterns on the Plasma Membrane
- PMID: 27603951
- PMCID: PMC5014321
- DOI: 10.1371/journal.pcbi.1005095
Inhomogeneity Based Characterization of Distribution Patterns on the Plasma Membrane
Abstract
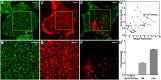
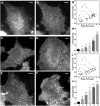
Cell surface protein and lipid molecules are organized in various patterns: randomly, along gradients, or clustered when segregated into discrete micro- and nano-domains. Their distribution is tightly coupled to events such as polarization, endocytosis, and intracellular signaling, but challenging to quantify using traditional techniques. Here we present a novel approach to quantify the distribution of plasma membrane proteins and lipids. This approach describes spatial patterns in degrees of inhomogeneity and incorporates an intensity-based correction to analyze images with a wide range of resolutions; we have termed it Quantitative Analysis of the Spatial distributions in Images using Mosaic segmentation and Dual parameter Optimization in Histograms (QuASIMoDOH). We tested its applicability using simulated microscopy images and images acquired by widefield microscopy, total internal reflection microscopy, structured illumination microscopy, and photoactivated localization microscopy. We validated QuASIMoDOH, successfully quantifying the distribution of protein and lipid molecules detected with several labeling techniques, in different cell model systems. We also used this method to characterize the reorganization of cell surface lipids in response to disrupted endosomal trafficking and to detect dynamic changes in the global and local organization of epidermal growth factor receptors across the cell surface. Our findings demonstrate that QuASIMoDOH can be used to assess protein and lipid patterns, quantifying distribution changes and spatial reorganization at the cell surface. An ImageJ/Fiji plugin of this analysis tool is provided.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Accurate cell segmentation in microscopy images using membrane patterns.Bioinformatics. 2014 Sep 15;30(18):2644-51. doi: 10.1093/bioinformatics/btu302. Epub 2014 May 21. Bioinformatics. 2014. PMID: 24849580
-
QSIM: quantitative structured illumination microscopy image processing in ImageJ.Biomed Eng Online. 2015 Jan 14;14:4. doi: 10.1186/1475-925X-14-4. Biomed Eng Online. 2015. PMID: 25588495 Free PMC article.
-
Quantitative analysis of the heterogeneous population of endocytic vesicles.J Bioinform Comput Biol. 2017 Apr;15(2):1750008. doi: 10.1142/S0219720017500081. Epub 2017 Mar 7. J Bioinform Comput Biol. 2017. PMID: 28351215
-
Quantifying spatial and temporal variations of the cell membrane ultra-structure by bimFCS.Methods. 2018 May 1;140-141:151-160. doi: 10.1016/j.ymeth.2018.02.019. Epub 2018 Mar 9. Methods. 2018. PMID: 29530504 Free PMC article. Review.
-
Analyzing Protein Clusters on the Plasma Membrane: Application of Spatial Statistical Analysis Methods on Super-Resolution Microscopy Images.Adv Anat Embryol Cell Biol. 2016;219:95-122. doi: 10.1007/978-3-319-28549-8_4. Adv Anat Embryol Cell Biol. 2016. PMID: 27207364 Review.
Cited by
-
Ground state depletion microscopy as a tool for studying microglia-synapse interactions.J Neurosci Res. 2021 Jun;99(6):1515-1532. doi: 10.1002/jnr.24819. Epub 2021 Mar 7. J Neurosci Res. 2021. PMID: 33682204 Free PMC article.
-
NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis.EMBO J. 2024 Apr;43(7):1164-1186. doi: 10.1038/s44318-024-00055-y. Epub 2024 Feb 23. EMBO J. 2024. PMID: 38396301 Free PMC article.
-
Structural basis of NINJ1-mediated plasma membrane rupture in cell death.Nature. 2023 Jun;618(7967):1065-1071. doi: 10.1038/s41586-023-05991-z. Epub 2023 May 17. Nature. 2023. PMID: 37198476 Free PMC article.
-
Molecular Imaging of HER2 in Patient Tissues with Touch Prep-Quantitative Single Molecule Localization Microscopy.Methods Mol Biol. 2022;2394:231-248. doi: 10.1007/978-1-0716-1811-0_15. Methods Mol Biol. 2022. PMID: 35094332 Free PMC article.
-
Super-resolution optical microscopy for studying membrane structure and dynamics.J Phys Condens Matter. 2017 Jul 12;29(27):273001. doi: 10.1088/1361-648X/aa7185. Epub 2017 May 8. J Phys Condens Matter. 2017. PMID: 28481213 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

