Fragment-Based Drug Design Facilitated by Protein-Templated Click Chemistry: Fragment Linking and Optimization of Inhibitors of the Aspartic Protease Endothiapepsin
- PMID: 27604032
- PMCID: PMC5095814
- DOI: 10.1002/chem.201603001
Fragment-Based Drug Design Facilitated by Protein-Templated Click Chemistry: Fragment Linking and Optimization of Inhibitors of the Aspartic Protease Endothiapepsin
Abstract
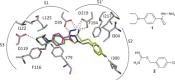
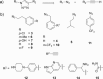
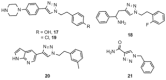
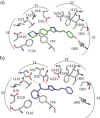
There is an urgent need for the development of efficient methodologies that accelerate drug discovery. We demonstrate that the strategic combination of fragment linking/optimization and protein-templated click chemistry is an efficient and powerful method that accelerates the hit-identification process for the aspartic protease endothiapepsin. The best binder, which inhibits endothiapepsin with an IC50 value of 43 μm, represents the first example of triazole-based inhibitors of endothiapepsin. Our strategy could find application on a whole range of drug targets.
Keywords: click chemistry; drug design; enzymes; inhibitors; liquid chromatography.
© 2016 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

Similar articles
-
Fragment Linking and Optimization of Inhibitors of the Aspartic Protease Endothiapepsin: Fragment-Based Drug Design Facilitated by Dynamic Combinatorial Chemistry.Angew Chem Int Ed Engl. 2016 Aug 1;55(32):9422-6. doi: 10.1002/anie.201603074. Epub 2016 Jul 12. Angew Chem Int Ed Engl. 2016. PMID: 27400756 Free PMC article.
-
Structure-Based Optimization of Inhibitors of the Aspartic Protease Endothiapepsin.Int J Mol Sci. 2015 Aug 14;16(8):19184-94. doi: 10.3390/ijms160819184. Int J Mol Sci. 2015. PMID: 26287174 Free PMC article.
-
Structure-based design of inhibitors of the aspartic protease endothiapepsin by exploiting dynamic combinatorial chemistry.Angew Chem Int Ed Engl. 2014 Mar 17;53(12):3259-63. doi: 10.1002/anie.201309682. Epub 2014 Feb 14. Angew Chem Int Ed Engl. 2014. PMID: 24532096
-
Aspartic proteases in drug discovery.Curr Pharm Des. 2007;13(3):271-85. doi: 10.2174/138161207779313560. Curr Pharm Des. 2007. PMID: 17313361 Review.
-
Inhibitors of aspartic proteases in human diseases: molecular modeling comes of age.Pharmazie. 1999 May;54(5):319-29. Pharmazie. 1999. PMID: 10368824 Review.
Cited by
-
Protein-Templated Fragment Ligations-From Molecular Recognition to Drug Discovery.Angew Chem Int Ed Engl. 2017 Jun 19;56(26):7358-7378. doi: 10.1002/anie.201610372. Epub 2017 May 31. Angew Chem Int Ed Engl. 2017. PMID: 28117936 Free PMC article. Review.
-
Diradical-singlet character of 1,3-dipoles affects reactivity of 1,3-dipolar cycloaddition reactions and intramolecular cyclization.J Mol Model. 2019 Sep 7;25(10):306. doi: 10.1007/s00894-019-4162-9. J Mol Model. 2019. PMID: 31494732
-
Proteintemplat-gesteuerte Fragmentligationen - von der molekularen Erkennung zur Wirkstofffindung.Angew Chem Weinheim Bergstr Ger. 2017 Jun 19;129(26):7464-7485. doi: 10.1002/ange.201610372. Epub 2017 May 31. Angew Chem Weinheim Bergstr Ger. 2017. PMID: 32313319 Free PMC article.
References
-
- Erlanson D. A., Mcdowell R. S., Brien T. O., J. Med. Chem. 2004, 47, 3463–3482. - PubMed
-
- Hajduk P. J., Greer J., Nat. Rev. Drug Discovery 2007, 6, 211–219. - PubMed
-
- Shuker S. B., Hajduk P. J., Meadows R. P., Fesik S. W., Science 1996, 274, 1531–1534. - PubMed
-
- Erlanson D. A., Top. Curr. Chem. 2011, 317, 1–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

