Microdialysate concentration changes do not provide sufficient information to evaluate metabolic effects of lactate supplementation in brain-injured patients
- PMID: 27604313
- PMCID: PMC5094313
- DOI: 10.1177/0271678X16666552
Microdialysate concentration changes do not provide sufficient information to evaluate metabolic effects of lactate supplementation in brain-injured patients
Abstract
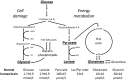
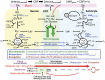
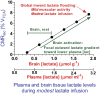
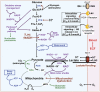
Cerebral microdialysis is a widely used clinical tool for monitoring extracellular concentrations of selected metabolites after brain injury and to guide neurocritical care. Extracellular glucose levels and lactate/pyruvate ratios have high diagnostic value because they can detect hypoglycemia and deficits in oxidative metabolism, respectively. In addition, patterns of metabolite concentrations can distinguish between ischemia and mitochondrial dysfunction, and are helpful to choose and evaluate therapy. Increased intracranial pressure can be life-threatening after brain injury, and hypertonic solutions are commonly used for pressure reduction. Recent reports have advocated use of hypertonic sodium lactate, based on claims that it is glucose sparing and provides an oxidative fuel for injured brain. However, changes in extracellular concentrations in microdialysate are not evidence that a rise in extracellular glucose level is beneficial or that lactate is metabolized and improves neuroenergetics. The increase in glucose concentration may reflect inhibition of glycolysis, glycogenolysis, and pentose phosphate shunt pathway fluxes by lactate flooding in patients with mitochondrial dysfunction. In such cases, lactate will not be metabolizable and lactate flooding may be harmful. More rigorous approaches are required to evaluate metabolic and physiological effects of administration of hypertonic sodium lactate to brain-injured patients.
Keywords: Cerebral microdialysis; brain metabolism; glucose; lactate supplementation; traumatic brain injury.
© The Author(s) 2016.
Figures


Similar articles
-
Improvement of Neuroenergetics by Hypertonic Lactate Therapy in Patients with Traumatic Brain Injury Is Dependent on Baseline Cerebral Lactate/Pyruvate Ratio.J Neurotrauma. 2016 Apr 1;33(7):681-7. doi: 10.1089/neu.2015.4057. Epub 2015 Dec 15. J Neurotrauma. 2016. PMID: 26421521 Free PMC article. Clinical Trial.
-
Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain.Intensive Care Med. 2014 Mar;40(3):412-21. doi: 10.1007/s00134-013-3203-6. Epub 2014 Jan 30. Intensive Care Med. 2014. PMID: 24477453 Clinical Trial.
-
Hypertonic Lactate to Improve Cerebral Perfusion and Glucose Availability After Acute Brain Injury.Crit Care Med. 2018 Oct;46(10):1649-1655. doi: 10.1097/CCM.0000000000003274. Crit Care Med. 2018. PMID: 29923931
-
Lactate and the injured brain: friend or foe?Curr Opin Crit Care. 2014 Apr;20(2):133-40. doi: 10.1097/MCC.0000000000000072. Curr Opin Crit Care. 2014. PMID: 24561705 Review.
-
Cerebral Lactate Metabolism After Traumatic Brain Injury.Curr Neurol Neurosci Rep. 2016 Apr;16(4):31. doi: 10.1007/s11910-016-0638-5. Curr Neurol Neurosci Rep. 2016. PMID: 26898683 Review.
Cited by
-
The Effects of Temperature Management on Brain Microcirculation, Oxygenation and Metabolism.Brain Sci. 2022 Oct 21;12(10):1422. doi: 10.3390/brainsci12101422. Brain Sci. 2022. PMID: 36291355 Free PMC article.
-
Lactate in the brain: from metabolic end-product to signalling molecule.Nat Rev Neurosci. 2018 Apr;19(4):235-249. doi: 10.1038/nrn.2018.19. Epub 2018 Mar 8. Nat Rev Neurosci. 2018. PMID: 29515192 Review.
-
Nutrition Therapy, Glucose Control, and Brain Metabolism in Traumatic Brain Injury: A Multimodal Monitoring Approach.Front Neurosci. 2020 Mar 24;14:190. doi: 10.3389/fnins.2020.00190. eCollection 2020. Front Neurosci. 2020. PMID: 32265626 Free PMC article. Review.
-
The longitudinal biochemical profiling of TBI in a drop weight model of TBI.Sci Rep. 2023 Dec 14;13(1):22260. doi: 10.1038/s41598-023-48539-x. Sci Rep. 2023. PMID: 38097614 Free PMC article.
-
High-physiological and supra-physiological 1,2-13C2 glucose focal supplementation to the traumatised human brain.J Cereb Blood Flow Metab. 2023 Oct;43(10):1685-1701. doi: 10.1177/0271678X231173584. Epub 2023 May 8. J Cereb Blood Flow Metab. 2023. PMID: 37157814 Free PMC article.
References
-
- Nordström CH, Nielsen TH, Jacobsen A. Techniques and strategies in neurocritical care originating from southern Scandinavia. J Rehabil Med 2013; 45: 710–717. - PubMed
-
- Nordström CH. Cerebral energy metabolism and microdialysis in neurocritical care. Childs Nerv Syst 2010; 26: 465–472. - PubMed
-
- Reinstrup P, Ståhl N, Mellergård P, et al. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery 2000; 47: 701–710. - PubMed
-
- Amer-Wahlin I, Nord A, Bottalico B, et al. Fetal cerebral energy metabolism and electrocardiogram during experimental umbilical cord occlusion and resuscitation. J Matern Fetal Neonatal Med 2010; 23: 158–166. - PubMed
-
- Nielsen TH, Olsen NV, Toft P, et al. Cerebral energy metabolism during mitochondrial dysfunction induced by cyanide in piglets. Acta Anaesthesiol Scand 2013; 57: 793–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

