Osteogenic Differentiation of Mesenchymal Stem Cells by Mimicking the Cellular Niche of the Endochondral Template
- PMID: 27604384
- PMCID: PMC5073234
- DOI: 10.1089/ten.TEA.2015.0339
Osteogenic Differentiation of Mesenchymal Stem Cells by Mimicking the Cellular Niche of the Endochondral Template
Abstract
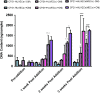
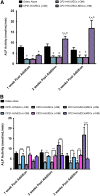
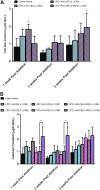
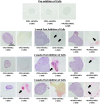
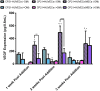
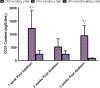
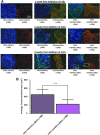
In vitro bone regeneration strategies that prime mesenchymal stem cells (MSCs) with chondrogenic factors, to mimic aspects of the endochondral ossification process, have been shown to promote mineralization and vascularization by MSCs both in vitro and when implanted in vivo. However, these approaches required the use of osteogenic supplements, namely dexamethasone, ascorbic acid, and β-glycerophosphate, none of which are endogenous mediators of bone formation in vivo. Rather MSCs, endothelial progenitor cells, and chondrocytes all reside in proximity within the cartilage template and might paracrineally regulate osteogenic differentiation. Thus, this study tests the hypothesis that an in vitro bone regeneration approach that mimics the cellular niche existing during endochondral ossification, through coculture of MSCs, endothelial cells, and chondrocytes, will obviate the need for extraneous osteogenic supplements and provide an alternative strategy to elicit osteogenic differentiation of MSCs and mineral production. The specific objectives of this study were to (1) mimic the cellular niche existing during endochondral ossification and (2) investigate whether osteogenic differentiation could be induced without the use of any external growth factors. To test the hypothesis, we evaluated the mineralization and vessel formation potential of (a) a novel methodology involving both chondrogenic priming and the coculture of human umbilical vein endothelial cells (HUVECs) and MSCs compared with (b) chondrogenic priming of MSCs alone, (c) addition of HUVECs to chondrogenically primed MSC aggregates, (d-f) the same experimental groups cultured in the presence of osteogenic supplements and (g) a noncoculture group cultured in the presence of osteogenic growth factors alone. Biochemical (DNA, alkaline phosphatase [ALP], calcium, CD31+, vascular endothelial growth factor [VEGF]), histological (alcian blue, alizarin red), and immunohistological (CD31+) analyses were conducted to investigate osteogenic differentiation and vascularization at various time points (1, 2, and 3 weeks). The coculture methodology enhanced both osteogenesis and vasculogenesis compared with osteogenic differentiation alone, whereas osteogenic supplements inhibited the osteogenesis and vascularization (ALP, calcium, and VEGF) induced through coculture alone. Taken together, these results suggest that chondrogenic and vascular priming can obviate the need for osteogenic supplements to induce osteogenesis of human MSCs in vitro, while allowing for the formation of rudimentary vessels.
Conflict of interest statement
Statement No competing financial interests exist.
Figures

Similar articles
-
An in vitro bone tissue regeneration strategy combining chondrogenic and vascular priming enhances the mineralization potential of mesenchymal stem cells in vitro while also allowing for vessel formation.Tissue Eng Part A. 2015 Apr;21(7-8):1320-32. doi: 10.1089/ten.TEA.2014.0249. Epub 2015 Mar 3. Tissue Eng Part A. 2015. PMID: 25588588
-
* Mimicking the Biochemical and Mechanical Extracellular Environment of the Endochondral Ossification Process to Enhance the In Vitro Mineralization Potential of Human Mesenchymal Stem Cells.Tissue Eng Part A. 2017 Dec;23(23-24):1466-1478. doi: 10.1089/ten.TEA.2017.0052. Epub 2017 Oct 12. Tissue Eng Part A. 2017. PMID: 28756737
-
Effects of in vitro endochondral priming and pre-vascularisation of human MSC cellular aggregates in vivo.Stem Cell Res Ther. 2015 Nov 5;6:218. doi: 10.1186/s13287-015-0210-2. Stem Cell Res Ther. 2015. PMID: 26541817 Free PMC article.
-
Impact of developmental origin, niche mechanics and oxygen availability on osteogenic differentiation capacity of mesenchymal stem/stromal cells.Acta Biochim Pol. 2019 Dec 29;66(4):491-498. doi: 10.18388/abp.2019_2893. Acta Biochim Pol. 2019. PMID: 31883439 Review.
-
Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues.Tissue Eng Part B Rev. 2021 Jun;27(3):199-214. doi: 10.1089/ten.TEB.2020.0132. Epub 2020 Sep 25. Tissue Eng Part B Rev. 2021. PMID: 32854589 Free PMC article. Review.
Cited by
-
New insights into the biomimetic design and biomedical applications of bioengineered bone microenvironments.APL Bioeng. 2021 Nov 3;5(4):041507. doi: 10.1063/5.0065152. eCollection 2021 Dec. APL Bioeng. 2021. PMID: 34765857 Free PMC article. Review.
-
Isolation and characterization of canine perivascular stem/stromal cells for bone tissue engineering.PLoS One. 2017 May 10;12(5):e0177308. doi: 10.1371/journal.pone.0177308. eCollection 2017. PLoS One. 2017. PMID: 28489940 Free PMC article.
-
Insights into Arbutin Effects on Bone Cells: Towards the Development of Antioxidant Titanium Implants.Antioxidants (Basel). 2020 Jul 2;9(7):579. doi: 10.3390/antiox9070579. Antioxidants (Basel). 2020. PMID: 32630762 Free PMC article.
-
Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells.Int J Nanomedicine. 2019 Aug 19;14:6615-6630. doi: 10.2147/IJN.S217245. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31695360 Free PMC article.
-
Biological and mechanical interplay at the Macro- and Microscales Modulates the Cell-Niche Fate.Sci Rep. 2018 Mar 2;8(1):3937. doi: 10.1038/s41598-018-21860-6. Sci Rep. 2018. PMID: 29500447 Free PMC article.
References
-
- Cancedda R., Giannoni P., and Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28, 4240, 2007 - PubMed
-
- Dawson J.I., and Oreffo R.O. Bridging the regeneration gap: stem cells, biomaterials and clinical translation in bone tissue engineering. Arch Biochem Biophys 473, 124, 2008 - PubMed
-
- Rose F.R., and Oreffo R.O. Bone tissue engineering: hope vs hype. Biochem Biophy Res Commun 292, 1, 2002 - PubMed
-
- Lyons F.G., Al-Munajjed A.A., Kieran S.M., Toner M.E., Murphy C.M., Duffy G.P., et al. . The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials 31, 9232, 2010 - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

