Space-time codependence of retinal ganglion cells can be explained by novel and separable components of their receptive fields
- PMID: 27604400
- PMCID: PMC5027358
- DOI: 10.14814/phy2.12952
Space-time codependence of retinal ganglion cells can be explained by novel and separable components of their receptive fields
Abstract
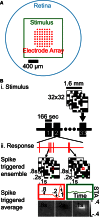
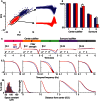
Reverse correlation methods such as spike-triggered averaging consistently identify the spatial center in the linear receptive fields (RFs) of retinal ganglion cells (GCs). However, the spatial antagonistic surround observed in classical experiments has proven more elusive. Tests for the antagonistic surround have heretofore relied on models that make questionable simplifying assumptions such as space-time separability and radial homogeneity/symmetry. We circumvented these, along with other common assumptions, and observed a linear antagonistic surround in 754 of 805 mouse GCs. By characterizing the RF's space-time structure, we found the overall linear RF's inseparability could be accounted for both by tuning differences between the center and surround and differences within the surround. Finally, we applied this approach to characterize spatial asymmetry in the RF surround. These results shed new light on the spatiotemporal organization of GC linear RFs and highlight a major contributor to its inseparability.
Keywords: Retinal ganglion cells; space–time separability; spatiotemporal tuning.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures




Similar articles
-
Spatiotemporal organization of the receptive fields of retinal ganglion cells in the cat: a phenomenological model.Acta Neurobiol Exp (Wars). 1986;46(2-3):153-69. Acta Neurobiol Exp (Wars). 1986. PMID: 3776708
-
Distinct subcomponents of mouse retinal ganglion cell receptive fields are differentially altered by light adaptation.Vision Res. 2017 Feb;131:96-105. doi: 10.1016/j.visres.2016.12.015. Epub 2017 Jan 24. Vision Res. 2017. PMID: 28087445
-
Retinal ganglion cells--spatial organization of the receptive field reduces temporal redundancy.Eur J Neurosci. 2008 Sep;28(5):914-23. doi: 10.1111/j.1460-9568.2008.06394.x. Epub 2008 Aug 8. Eur J Neurosci. 2008. PMID: 18691326 Free PMC article.
-
Features and functions of nonlinear spatial integration by retinal ganglion cells.J Physiol Paris. 2013 Nov;107(5):338-48. doi: 10.1016/j.jphysparis.2012.12.001. Epub 2012 Dec 20. J Physiol Paris. 2013. PMID: 23262113 Review.
-
Spatiotemporal inseparability in early visual processing.Biol Cybern. 1985;52(3):153-64. doi: 10.1007/BF00339944. Biol Cybern. 1985. PMID: 3896327 Review.
Cited by
-
Abnormal perception of pattern-induced flicker colors in subjects with glaucoma.J Vis. 2022 Feb 1;22(2):5. doi: 10.1167/jov.22.2.5. J Vis. 2022. PMID: 35133432 Free PMC article.
-
Glycinergic and GABAergic interneurons shift the location and differentially alter the size of ganglion cell receptive field centers in the mammalian retina.Vision Res. 2020 May;170:18-24. doi: 10.1016/j.visres.2020.03.002. Epub 2020 Mar 25. Vision Res. 2020. PMID: 32217368 Free PMC article.
-
Single transient intraocular pressure elevations cause prolonged retinal ganglion cell dysfunction and retinal capillary abnormalities in mice.Exp Eye Res. 2020 Dec;201:108296. doi: 10.1016/j.exer.2020.108296. Epub 2020 Oct 8. Exp Eye Res. 2020. PMID: 33039455 Free PMC article.
-
The ON Crossover Circuitry Shapes Spatiotemporal Profile in the Center and Surround of Mouse OFF Retinal Ganglion Cells.Front Neural Circuits. 2016 Dec 22;10:106. doi: 10.3389/fncir.2016.00106. eCollection 2016. Front Neural Circuits. 2016. PMID: 28066192 Free PMC article.
-
Intraocular Pressure Elevation Compromises Retinal Ganglion Cell Light Adaptation.Invest Ophthalmol Vis Sci. 2020 Oct 1;61(12):15. doi: 10.1167/iovs.61.12.15. Invest Ophthalmol Vis Sci. 2020. PMID: 33064129 Free PMC article.
References
-
- Alpern, M. , Fulton A. B., and Baker B. N.. 1987. “Self‐screening” of rhodopsin in rod outer segments. Vision. Res. 27:1459–1470. - PubMed
-
- Bloomfield, S. A. 1996. Effect of spike blockade on the receptive‐field size of amacrine and ganglion cells in the rabbit retina. J. Neurophysiol. 75:1878–1893. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

