Increased pulmonary arteriolar tone associated with lung oxidative stress and nitric oxide in a mouse model of Alzheimer's disease
- PMID: 27604401
- PMCID: PMC5027359
- DOI: 10.14814/phy2.12953
Increased pulmonary arteriolar tone associated with lung oxidative stress and nitric oxide in a mouse model of Alzheimer's disease
Abstract
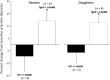
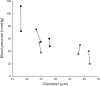
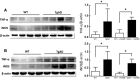
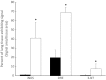
Vascular dysfunction and decreased cerebral blood flow are linked to Alzheimer's disease (AD). Loss of endothelial nitric oxide (NO) and oxidative stress in human cerebrovascular endothelium increase expression of amyloid precursor protein (APP) and enhance production of the Aβ peptide, suggesting that loss of endothelial NO contributes to AD pathology. We hypothesize that decreased systemic NO bioavailability in AD may also impact lung microcirculation and induce pulmonary endothelial dysfunction. The acute effect of NO synthase (NOS) inhibition on pulmonary arteriolar tone was assessed in a transgenic mouse model (TgAD) of AD (C57BL/6-Tg(Thy1-APPSwDutIowa)BWevn/Mmjax) and age-matched wild-type controls (C57BL/6J). Arteriolar diameters were measured before and after the administration of the NOS inhibitor, L-NAME Lung superoxide formation (DHE) and formation of nitrotyrosine (3-NT) were assessed as indicators of oxidative stress, inducible NOS (iNOS) and tumor necrosis factor alpha (TNF-α) expression as indicators of inflammation. Administration of L-NAME caused either significant pulmonary arteriolar constriction or no change from baseline tone in wild-type (WT) mice, and significant arteriolar dilation in TgAD mice. DHE, 3-NT, TNF-α, and iNOS expression were higher in TgAD lung tissue, compared to WT mice. These data suggest L-NAME could induce increased pulmonary arteriolar tone in WT mice from loss of bioavailable NO In contrast, NOS inhibition with L-NAME had a vasodilator effect in TgAD mice, potentially caused by decreased reactive nitrogen species formation, while significant oxidative stress and inflammation were present. We conclude that AD may increase pulmonary microvascular tone as a result of loss of bioavailable NO and increased oxidative stress. Our findings suggest that AD may have systemic microvascular implications beyond central neural control mechanisms.
Keywords: Amyloid precursor protein; endothelial dysfunction; lung microcirculation; neuroinflammation; reactive nitrogen species.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures




References
-
- Cheung, C. Y. , Ong Y. T., Ihram M. K., Ong S. Y., Li X., Hilal S., et al. 2014. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 10:135–142. - PubMed
-
- De la Torre, J. C. 2004. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma. and dialectics. Lancet Neurol. 3:184–190. - PubMed
-
- De la Torre, J. C. , and Stefano G. M.. 2000. Evidence that Alzheimer's disease is a microvascular disorder: the role of constitutive nitric oxide. Brain Res. Rev. 34:119–136. - PubMed
-
- DeCatarina, R. , Libby P., Peng H. B., Thannickal V. J., Rajavashisth T. B., Gimbrone M. A. Jr, et al. 1995. Nitric oxide decreases cytokine‐induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 96:60–68. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

