Placental Origins of Chronic Disease
- PMID: 27604528
- PMCID: PMC5504455
- DOI: 10.1152/physrev.00029.2015
Placental Origins of Chronic Disease
Abstract
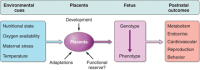
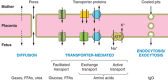
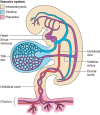
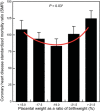
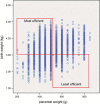
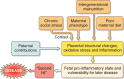
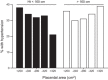
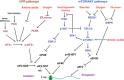
Epidemiological evidence links an individual's susceptibility to chronic disease in adult life to events during their intrauterine phase of development. Biologically this should not be unexpected, for organ systems are at their most plastic when progenitor cells are proliferating and differentiating. Influences operating at this time can permanently affect their structure and functional capacity, and the activity of enzyme systems and endocrine axes. It is now appreciated that such effects lay the foundations for a diverse array of diseases that become manifest many years later, often in response to secondary environmental stressors. Fetal development is underpinned by the placenta, the organ that forms the interface between the fetus and its mother. All nutrients and oxygen reaching the fetus must pass through this organ. The placenta also has major endocrine functions, orchestrating maternal adaptations to pregnancy and mobilizing resources for fetal use. In addition, it acts as a selective barrier, creating a protective milieu by minimizing exposure of the fetus to maternal hormones, such as glucocorticoids, xenobiotics, pathogens, and parasites. The placenta shows a remarkable capacity to adapt to adverse environmental cues and lessen their impact on the fetus. However, if placental function is impaired, or its capacity to adapt is exceeded, then fetal development may be compromised. Here, we explore the complex relationships between the placental phenotype and developmental programming of chronic disease in the offspring. Ensuring optimal placentation offers a new approach to the prevention of disorders such as cardiovascular disease, diabetes, and obesity, which are reaching epidemic proportions.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Acosta O, Ramirez VI, Lager S, Gaccioli F, Dudley DJ, Powell TL, Jansson T. Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am J Obstet Gynecol 212: e221–227, 2015. - PubMed
-
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007. - PubMed
-
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 250: 358–373, 2002. - PubMed
-
- Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med 41: 66–72, 2009. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

