Tn-MUC1 DC Vaccination of Rhesus Macaques and a Phase I/II Trial in Patients with Nonmetastatic Castrate-Resistant Prostate Cancer
- PMID: 27604597
- PMCID: PMC5331878
- DOI: 10.1158/2326-6066.CIR-15-0189
Tn-MUC1 DC Vaccination of Rhesus Macaques and a Phase I/II Trial in Patients with Nonmetastatic Castrate-Resistant Prostate Cancer
Abstract
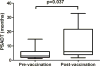
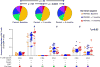
MUC1 is a glycoprotein expressed on the apical surface of ductal epithelial cells. Malignant transformation results in loss of polarization and overexpression of hypoglycosylated MUC1 carrying truncated carbohydrates known as T or Tn tumor antigens. Tumor MUC1 bearing Tn carbohydrates (Tn-MUC1) represent a potential target for immunotherapy. We evaluated the Tn-MUC1 glycopeptide in a human phase I/II clinical trial for safety that followed a preclinical study of different glycosylation forms of MUC1 in rhesus macaques, whose MUC1 is highly homologous to human MUC1. Either unglycosylated rhesus macaque MUC1 peptide (rmMUC1) or Tn-rmMUC1 glycopeptide was mixed with an adjuvant or loaded on autologous dendritic cells (DC), and responses were compared. Unglycosylated rmMUC1 peptide induced negligible humoral or cellular responses compared with the Tn-rmMUC1 glycopeptide. Tn-rmMUC1 loaded on DCs induced the highest anti-rmMUC1 T-cell responses and no clinical toxicity. In the phase I/II clinical study, 17 patients with nonmetastatic castrate-resistant prostate cancer (nmCRPC) were tested with a Tn-MUC1 glycopeptide-DC vaccine. Patients were treated with multiple intradermal and intranodal doses of autologous DCs, which were loaded with the Tn-MUC1 glycopeptide (and KLH as a positive control for immune reactivity). PSA doubling time (PSADT) improved significantly in 11 of 16 evaluable patients (P = 0.037). Immune response analyses detected significant Tn-MUC1-specific CD4+ and/or CD8+ T-cell intracellular cytokine responses in 5 out of 7 patients evaluated. In conclusion, vaccination with Tn-MUC1-loaded DCs in nmCRPC patients appears to be safe, able to induce significant T-cell responses, and have biological activity as measured by the increase in PSADT following vaccination. Cancer Immunol Res; 4(10); 881-92. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures



References
-
- Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der KT, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. - PubMed
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der KT, et al. EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79. - PubMed
-
- Cookson MS, Roth BJ, Dahm P, Engstrom C, Freedland SJ, Hussain M, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190:429–38. - PubMed
-
- Berthold DR, Pond GR, Soban F, de WR, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

