MYB107 and MYB9 Homologs Regulate Suberin Deposition in Angiosperms
- PMID: 27604696
- PMCID: PMC5059810
- DOI: 10.1105/tpc.16.00490
MYB107 and MYB9 Homologs Regulate Suberin Deposition in Angiosperms
Abstract
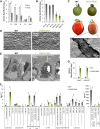
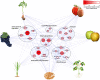
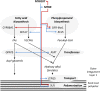
Suberin, a polymer composed of both aliphatic and aromatic domains, is deposited as a rough matrix upon plant surface damage and during normal growth in the root endodermis, bark, specialized organs (e.g., potato [Solanum tuberosum] tubers), and seed coats. To identify genes associated with the developmental control of suberin deposition, we investigated the chemical composition and transcriptomes of suberized tomato (Solanum lycopersicum) and russet apple (Malus x domestica) fruit surfaces. Consequently, a gene expression signature for suberin polymer assembly was revealed that is highly conserved in angiosperms. Seed permeability assays of knockout mutants corresponding to signature genes revealed regulatory proteins (i.e., AtMYB9 and AtMYB107) required for suberin assembly in the Arabidopsis thaliana seed coat. Seeds of myb107 and myb9 Arabidopsis mutants displayed a significant reduction in suberin monomers and altered levels of other seed coat-associated metabolites. They also exhibited increased permeability, and lower germination capacities under osmotic and salt stress. AtMYB9 and AtMYB107 appear to synchronize the transcriptional induction of aliphatic and aromatic monomer biosynthesis and transport and suberin polymerization in the seed outer integument layer. Collectively, our findings establish a regulatory system controlling developmentally deposited suberin, which likely differs from the one of stress-induced polymer assembly recognized to date.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures






References
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. - PubMed
-
- Bargel H., Koch K., Cerman Z., Neinhuis C. (2006). Structure–function relationships of the plant cuticle and cuticular waxes–a smart material? Funct. Plant Biol. 33: 893–910. - PubMed
-
- Beisson F., Li-Beisson Y., Pollard M. (2012). Solving the puzzles of cutin and suberin polymer biosynthesis. Curr. Opin. Plant Biol. 15: 329–337. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

