Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection
- PMID: 27604795
- PMCID: PMC5016293
- DOI: 10.3904/kjim.2016.166
Endoscopic gastritis, serum pepsinogen assay, and Helicobacter pylori infection
Abstract
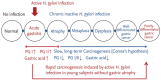
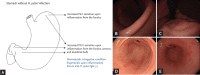
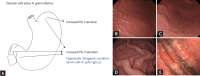
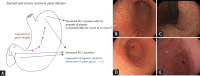
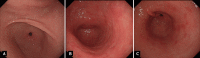
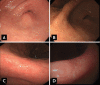
Endoscopic findings of the background gastric mucosa are important in the Helicobacter pylori-seroprevalent population. It is strongly correlated not only with the risk of gastric cancer, but also with the excretion ability of gastric mucosa cells. In noninfected subjects, common endoscopic findings are regular arrangement of collecting venules, chronic superficial gastritis, and erosive gastritis. In cases of active H. pylori infection, nodularity on the antrum, hemorrhagic spots on the fundus, and thickened gastric folds are common endoscopic findings. The secreting ability of the gastric mucosa cells is usually intact in both noninfected and actively infected stomachs, and the intragastric condition becomes hyperacidic upon inflammation. Increased serum pepsinogen II concentration correlates well with active H. pylori infection, and also indicates an increased risk of diffuse-type gastric cancer. In chronic inactive H. pylori infection, metaplastic gastritis and atrophic gastritis extending from the antrum (closed-type chronic atrophic gastritis) toward the corpus (open-type chronic atrophic gastritis) are common endoscopic findings. The intragastric environment is hypoacidic and the risk of intestinal-type gastric cancer is increased in such conditions. Furthermore, there is a decrease in serum pepsinogen I concentration when the secreting ability of the gastric mucosa cells is damaged. Serologic and endoscopic changes that occur upon H. pylori infection are important findings for estimating the secreting ability of the gastric mucosa cells, and could be applied for the secondary prevention of gastric cancer.
Keywords: Atrophy; Endoscopy; Gastritis; Helicobacter pylori; Pepsinogens.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer.Int J Cancer. 2014 Mar 15;134(6):1445-57. doi: 10.1002/ijc.28470. Epub 2013 Oct 3. Int J Cancer. 2014. PMID: 24009139
-
Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio--a possible novel and non-invasive marker for gastric atrophy.Hepatogastroenterology. 2004 Sep-Oct;51(59):1249-54. Hepatogastroenterology. 2004. PMID: 15362725
-
Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study.Scand J Gastroenterol. 2002 Jul;37(7):785-91. Scand J Gastroenterol. 2002. PMID: 12190091
-
Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis.World J Gastroenterol. 2020 Feb 7;26(5):466-477. doi: 10.3748/wjg.v26.i5.466. World J Gastroenterol. 2020. PMID: 32089624 Free PMC article. Review.
-
Usefulness of magnifying narrow-band imaging endoscopy in the Helicobacter pylori-related chronic gastritis.Digestion. 2011;83(3):161-6. doi: 10.1159/000321799. Epub 2011 Jan 21. Digestion. 2011. PMID: 21266810 Review.
Cited by
-
The Diagnostic Value of Serum Gastrin-17 and Pepsinogen for Gastric Cancer Screening in Eastern China.Gastroenterol Res Pract. 2021 Apr 12;2021:6894248. doi: 10.1155/2021/6894248. eCollection 2021. Gastroenterol Res Pract. 2021. PMID: 33936196 Free PMC article.
-
Effectiveness of serological markers of gastric mucosal atrophy in the gastric precancer screening and in cancer prevention.World J Gastrointest Endosc. 2024 Aug 16;16(8):462-471. doi: 10.4253/wjge.v16.i8.462. World J Gastrointest Endosc. 2024. PMID: 39155993 Free PMC article.
-
Diagnostic performance of serum pepsinogen assay for the prediction of atrophic gastritis and gastric neoplasms: Protocol for a systematic review and meta-analysis.Medicine (Baltimore). 2019 Jan;98(4):e14240. doi: 10.1097/MD.0000000000014240. Medicine (Baltimore). 2019. PMID: 30681610 Free PMC article.
-
Gastric Cancer Screening by Combined Determination of Serum Helicobacter pylori Antibody and Pepsinogen Concentrations: ABC Method for Gastric Cancer Screening.Chin Med J (Engl). 2018 May 20;131(10):1232-1239. doi: 10.4103/0366-6999.231512. Chin Med J (Engl). 2018. PMID: 29722342 Free PMC article. Review.
-
ABC Classification Is Less Useful for Older Koreans Born before 1960.Gut Liver. 2019 Sep 15;13(5):522-530. doi: 10.5009/gnl18399. Gut Liver. 2019. PMID: 30970432 Free PMC article.
References
-
- Chu S, Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2012;28:587–593. - PubMed
-
- Ghosh T, Lewis DI, Axon AT, Everett SM. Review article: methods of measuring gastric acid secretion. Aliment Pharmacol Ther. 2011;33:768–781. - PubMed
-
- Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2–10. - PubMed
-
- Rehfeld JF, Bardram L, Hilsted L, Poitras P, Goetze JP. Pitfalls in diagnostic gastrin measurements. Clin Chem. 2012;58:831–836. - PubMed
-
- Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
