TGF-β Effects on Prostate Cancer Cell Migration and Invasion Require FosB
- PMID: 27604827
- PMCID: PMC5286811
- DOI: 10.1002/pros.23250
TGF-β Effects on Prostate Cancer Cell Migration and Invasion Require FosB
Abstract
Background: Activator Protein-1 (AP-1) family (cJun, JunB, JunD, cFos, FosB, Fra1, and Fra2) plays a central role in the transcriptional regulation of many genes that are associated with cell proliferation, differentiation, migration, metastasis, and survival. Many oncogenic signaling pathways converge at the AP-1 transcription complex. Transforming growth factor beta (TGF-β) is a multifunctional regulatory cytokine that regulates many aspects of cellular function, including cellular proliferation, differentiation, migration, apoptosis, adhesion, angiogenesis, immune surveillance, and survival.
Methods: This study investigated, the role of FOS proteins in TGF-β signaling in prostate cancer cell proliferation, migration, and invasion. Steady state expression levels of FOS mRNA and proteins were determined using RT-PCR and western blotting analyses. DU145 and PC3 prostate cancer cells were exposed to TGF-β1 at varying time and dosage, RT-PCR, western blot, and immunofluorescence analyses were used to determine TGF-β1 effect on FOS mRNA and protein expression levels as well as FosB subcellular localization. Transient silencing of FosB protein was used to determine its role in cell proliferation, migration, and invasion.
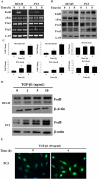
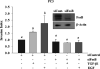
Results: Our data show that FOS mRNA and proteins were differentially expressed in human prostate epithelial (RWPE-1) and prostate cancer cell lines (LNCaP, DU145, and PC3). TGF-β1 induced the expression of FosB at both the mRNA and protein levels in DU145 and PC3 cells, whereas cFos and Fra1 were unaffected. Immunofluorescence analysis showed an increase in the accumulation of FosB protein in the nucleus of PC3 cells after treatment with exogenous TGF-β1. Selective knockdown of endogenous FosB by specific siRNA did not have any effect on cell proliferation in PC3 and DU145 cells. However, basal and TGF-β1- and EGF-induced cell migration was significantly reduced in DU145 and PC3 cells lacking endogenous FosB. TGF-β1- and EGF-induced cell invasion were also significantly decreased after FosB knockdown in PC3 cells.
Conclusion: Our data suggest that FosB is required for migration and invasion in prostate cancer cells. We also conclude that TGF-β1 effect on prostate cancer cell migration and invasion may be mediated through the induction of FosB. Prostate 77:72-81, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: AP-1; FosB; TGF-β; cell invasion; cell migration; prostate cancer.
© 2016 Wiley Periodicals, Inc.
Figures
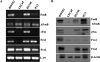
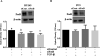
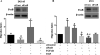
References
-
- DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics. CA Cancer J Clin. 2014;64:252–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

