SUMOylation regulates the intracellular fate of ZO-2
- PMID: 27604867
- PMCID: PMC11107645
- DOI: 10.1007/s00018-016-2352-5
SUMOylation regulates the intracellular fate of ZO-2
Abstract
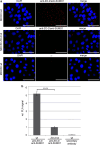
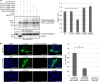
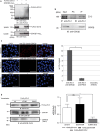
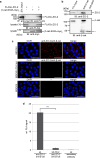
The zonula occludens (ZO)-2 protein links tight junctional transmembrane proteins to the actin cytoskeleton and associates with splicing and transcription factors in the nucleus. Multiple posttranslational modifications control the intracellular distribution of ZO-2. Here, we report that ZO-2 is a target of the SUMOylation machinery and provide evidence on how this modification may affect its cellular distribution and function. We show that ZO-2 associates with the E2 SUMO-conjugating enzyme Ubc9 and with SUMO-deconjugating proteases SENP1 and SENP3. In line with this, modification of ZO-2 by endogenous SUMO1 was detectable. Ubc9 fusion-directed SUMOylation confirmed SUMOylation of ZO-2 and was inhibited in the presence of SENP1 but not by an enzymatic-dead SENP1 protein. Moreover, lysine 730 in human ZO-2 was identified as a potential modification site. Mutation of this site to arginine resulted in prolonged nuclear localization of ZO-2 in nuclear recruitment assays. In contrast, a construct mimicking constitutive SUMOylation of ZO-2 (SUMO1ΔGG-ZO-2) was preferentially localized in the cytoplasm. Based on previous findings the differential localization of these ZO-2 constructs may affect glycogen-synthase-kinase-3β (GSK3β) activity and β-catenin/TCF-4-mediated transcription. In this context we observed that ZO-2 directly binds to GSK3β and SUMO1ΔGG-ZO-2 modulates its kinase activity. Moreover, we show that ZO-2 forms a complex with β-catenin. Wild-type ZO-2 and ZO-2-K730R inhibited transcriptional activity in reporter gene assays, whereas the cytosolic SUMO1ΔGG-ZO-2 did not. From these data we conclude that SUMOylation affects the intracellular localization of ZO-2 and its regulatory role on GSK3β and β-catenin signaling activity.
Keywords: Glycogen-synthase-kinase-3β; Occludin; Tight junction; Zonula occludens-2; β-Catenin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Tight junction protein ZO-2 modulates the nuclear accumulation of transcription factor TEAD.Mol Biol Cell. 2021 Jul 15;32(15):1347-1358. doi: 10.1091/mbc.E20-07-0470. Epub 2021 May 19. Mol Biol Cell. 2021. PMID: 34010016 Free PMC article.
-
The intracellular fate of zonula occludens 2 is regulated by the phosphorylation of SR repeats and the phosphorylation/O-GlcNAcylation of S257.Mol Biol Cell. 2013 Aug;24(16):2528-43. doi: 10.1091/mbc.E13-04-0224. Epub 2013 Jun 26. Mol Biol Cell. 2013. PMID: 23804652 Free PMC article.
-
Dynamic changes in sumoylation related proteins SUMO1, SENP1, and UBC9 during the peri implantation period in mice.Sci Rep. 2025 Jul 1;15(1):21497. doi: 10.1038/s41598-025-07677-0. Sci Rep. 2025. PMID: 40593246 Free PMC article.
-
ZO-2 Is a Master Regulator of Gene Expression, Cell Proliferation, Cytoarchitecture, and Cell Size.Int J Mol Sci. 2019 Aug 24;20(17):4128. doi: 10.3390/ijms20174128. Int J Mol Sci. 2019. PMID: 31450555 Free PMC article. Review.
-
Enhanced detection of in vivo SUMO conjugation by Ubc9 fusion-dependent sumoylation (UFDS).Methods Mol Biol. 2009;497:63-79. doi: 10.1007/978-1-59745-566-4_5. Methods Mol Biol. 2009. PMID: 19107411 Review.
Cited by
-
Polyubiquitination and SUMOylation Sites Regulate the Stability of ZO-2 Protein and the Sealing of Tight Junctions.Cells. 2022 Oct 19;11(20):3296. doi: 10.3390/cells11203296. Cells. 2022. PMID: 36291162 Free PMC article.
-
Zonula occludens 2 and Cell-Cell Contacts Are Required for Normal Nuclear Shape in Epithelia.Cells. 2021 Sep 28;10(10):2568. doi: 10.3390/cells10102568. Cells. 2021. PMID: 34685547 Free PMC article.
-
Apoptotic Fragmentation of Tricellulin.Int J Mol Sci. 2019 Oct 1;20(19):4882. doi: 10.3390/ijms20194882. Int J Mol Sci. 2019. PMID: 31581480 Free PMC article.
-
Tight junction protein ZO-2 modulates the nuclear accumulation of transcription factor TEAD.Mol Biol Cell. 2021 Jul 15;32(15):1347-1358. doi: 10.1091/mbc.E20-07-0470. Epub 2021 May 19. Mol Biol Cell. 2021. PMID: 34010016 Free PMC article.
-
The Rosetta Stone Hypothesis-Based Interaction of the Tumor Suppressor Proteins Nit1 and Fhit.Cells. 2023 Jan 17;12(3):353. doi: 10.3390/cells12030353. Cells. 2023. PMID: 36766695 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

