Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Enriches Regulatory T Cells via STAT6-Mediated Upregulation of TGF-β
- PMID: 27605008
- PMCID: PMC5101135
- DOI: 10.4049/jimmunol.1501628
Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Enriches Regulatory T Cells via STAT6-Mediated Upregulation of TGF-β
Abstract
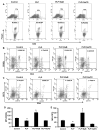
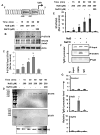
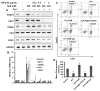
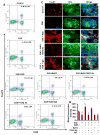
Upregulation and/or maintenance of regulatory T cells (Tregs) during autoimmune insults may have therapeutic efficacy in autoimmune diseases. Earlier we have reported that sodium benzoate (NaB), a metabolite of cinnamon and a Food and Drug Administration-approved drug against urea cycle disorders, upregulates Tregs and protects mice from experimental allergic encephalomyelitis, an animal model of multiple sclerosis. However, mechanisms by which NaB increases Tregs are poorly understood. Because TGF-β is an important inducer of Tregs, we examined the effect of NaB on the status of TGF-β. In this study, we demonstrated that NaB induced the expression of TGF-β mRNA and protein in normal as well as proteolipid protein-primed splenocytes. The presence of a consensus STAT6 binding site in the promoter of the TGF-β gene, activation of STAT6 in splenocytes by NaB, recruitment of STAT6 to the TGF-β promoter by NaB, and abrogation of NaB-induced expression of TGF-β in splenocytes by small interfering RNA knockdown of STAT6 suggest that NaB induces the expression of TGF-β via activation of STAT6. Furthermore, we demonstrated that blocking of TGF-β by neutralizing Abs abrogated NaB-mediated protection of Tregs and experimental allergic encephalomyelitis. These studies identify a new function of NaB in upregulating TGF-β via activation of STAT6, which may be beneficial in MS patients.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures





Similar articles
-
Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Ciliary Neurotrophic Factor in Astrocytes and Oligodendrocytes.Neurochem Res. 2015 Nov;40(11):2333-47. doi: 10.1007/s11064-015-1723-x. Epub 2015 Sep 23. Neurochem Res. 2015. PMID: 26399250 Free PMC article.
-
Sodium benzoate, a food additive and a metabolite of cinnamon, modifies T cells at multiple steps and inhibits adoptive transfer of experimental allergic encephalomyelitis.J Immunol. 2007 Jul 1;179(1):275-83. doi: 10.4049/jimmunol.179.1.275. J Immunol. 2007. PMID: 17579047 Free PMC article.
-
Cinnamon ameliorates experimental allergic encephalomyelitis in mice via regulatory T cells: implications for multiple sclerosis therapy.PLoS One. 2015 Jan 8;10(1):e0116566. doi: 10.1371/journal.pone.0116566. eCollection 2015. PLoS One. 2015. PMID: 25569428 Free PMC article.
-
TGF-β regulation of encephalitogenic and regulatory T cells in multiple sclerosis.Eur J Immunol. 2017 Mar;47(3):446-453. doi: 10.1002/eji.201646716. Epub 2017 Feb 10. Eur J Immunol. 2017. PMID: 28102541 Free PMC article. Review.
-
Th17 Cells and autoimmune encephalomyelitis (EAE/MS).Allergol Int. 2008 Jun;57(2):115-20. doi: 10.2332/allergolint.R-07-159. Allergol Int. 2008. PMID: 18427164 Review.
Cited by
-
Bixin Protects Against Kidney Interstitial Fibrosis Through Promoting STAT6 Degradation.Front Cell Dev Biol. 2020 Nov 17;8:576988. doi: 10.3389/fcell.2020.576988. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33313043 Free PMC article.
-
Your Regulatory T Cells Are What You Eat: How Diet and Gut Microbiota Affect Regulatory T Cell Development.Front Nutr. 2022 Apr 20;9:878382. doi: 10.3389/fnut.2022.878382. eCollection 2022. Front Nutr. 2022. PMID: 35529463 Free PMC article. Review.
-
Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756.Neurochem Res. 2017 Nov;42(11):3279-3288. doi: 10.1007/s11064-017-2367-9. Epub 2017 Aug 5. Neurochem Res. 2017. PMID: 28780732
-
Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Improves Cognitive Functions in Mice after Controlled Cortical Impact Injury.Int J Mol Sci. 2021 Dec 24;23(1):192. doi: 10.3390/ijms23010192. Int J Mol Sci. 2021. PMID: 35008615 Free PMC article.
-
Nebulization of RNS60, a Physically-Modified Saline, Attenuates the Adoptive Transfer of Experimental Allergic Encephalomyelitis in Mice: Implications for Multiple Sclerosis Therapy.Neurochem Res. 2017 May;42(5):1555-1570. doi: 10.1007/s11064-017-2214-z. Epub 2017 Mar 7. Neurochem Res. 2017. PMID: 28271325
References
-
- Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

