Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells
- PMID: 27605043
- PMCID: PMC5015093
- DOI: 10.1038/srep33132
Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells
Abstract
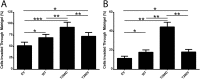
Calcium/calmodulin-stimulated protein kinase II (CaMKII) is a multi-functional kinase that controls a range of cellular functions, including proliferation, differentiation and apoptosis. The biological properties of CaMKII are regulated by multi-site phosphorylation. However, the role that CaMKII phosphorylation plays in cancer cell metastasis has not been examined. We demonstrate herein that CaMKII expression and phosphorylation at T286 is increased in breast cancer when compared to normal breast tissue, and that increased CAMK2 mRNA is associated with poor breast cancer patient prognosis (worse overall and distant metastasis free survival). Additionally, we show that overexpression of WT, T286D and T286V forms of CaMKII in MDA-MB-231 and MCF-7 breast cancer cells increases invasion, migration and anchorage independent growth, and that overexpression of the T286D phosphomimic leads to a further increase in the invasive, migratory and anchorage independent growth capacity of these cells. Pharmacological inhibition of CaMKII decreases MDA-MB-231 migration and invasion. Furthermore, we demonstrate that overexpression of T286D, but not WT or T286V-CaMKII, leads to phosphorylation of FAK, STAT5a, and Akt. These results demonstrate a novel function for phosphorylation of CaMKII at T286 in the control of breast cancer metastasis, offering a promising target for the development of therapeutics to prevent breast cancer metastasis.
Figures







References
-
- Ferlay J. et al. In IARC CancerBase No. 11 (Lyon, 2013).
-
- Kennecke H. et al. Metastatic behaviour of breast cancer subtypes. J Clin Oncol 28, 3271–3277 (2010). - PubMed
-
- Stewart T. A., Yapa K. T. & Monteith G. R. Altered calcium signaling in cancer cells. Biochim Biophys Acta 10.1016/j.bbamem.2014.08.016 (Epub ahead of print) (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

