New developments in anti-sickling agents: can drugs directly prevent the polymerization of sickle haemoglobin in vivo?
- PMID: 27605087
- PMCID: PMC5035193
- DOI: 10.1111/bjh.14264
New developments in anti-sickling agents: can drugs directly prevent the polymerization of sickle haemoglobin in vivo?
Abstract
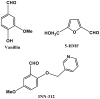
The hallmark of sickle cell disease is the polymerization of sickle haemoglobin due to a point mutation in the β-globin gene (HBB). Under low oxygen saturation, sickle haemoglobin assumes the tense (T-state) deoxygenated conformation that can form polymers, leading to rigid erythrocytes with impaired blood vessel transit, compounded or initiated by adhesion of erythrocytes to endothelium, neutrophils and platelets. This process results in vessel occlusion and ischaemia, with consequent acute pain, chronic organ damage, morbidity and mortality. Pharmacological agents that stabilize the higher oxygen affinity relaxed state (R-state) and/or destabilize the lower oxygen affinity T-state of haemoglobin have the potential to delay the sickling of circulating red cells by slowing polymerization kinetics. Relevant classes of agents include aromatic aldehydes, thiol derivatives, isothiocyanates and acyl salicylates derivatives. The aromatic aldehyde, 5-hydroxymethylfurfural (5-HMF) increases oxygen affinity of sickle haemoglobin and reduces hypoxia-induced sickling in vitro and protects sickle cell mice from effects of hypoxia. It has completed pre-clinical testing and has entered clinical trials as treatment for sickle cell disease. A related molecule, GBT440, has shown R-state stabilization and increased oxygen affinity in preclinical testing. Allosteric modifiers of haemoglobin as direct anti-sickling agents target the fundamental pathophysiological mechanism of sickle cell disease.
Keywords: 5-hydroxymethylfurfural; GBT440; TD-1; anti-sickling; haemoglobin allosteric effectors; sickle cell; vanillin.
© 2016 John Wiley & Sons Ltd.
Conflict of interest statement
Dr M.K. Safo is a co-owner of a patent for the use of 5-HMF in sickle cell disease, and receives research funding from AesRx, LLC, a licensee for 5-HMF (Aes-103/Bax-555). Dr G.J. Kato has collaborated with and received research funding from AesRx, LLC, through a Clinical Trials Agreement between AesRx, LLC and the National Heart, Lung and Blood Institute, and has received consulting fees from Baxalta, current holder of the license for 5-HMF (Aes-103/Bax-555), and research funding from Bayer HealthCare Pharmaceuticals Inc.
Figures


Similar articles
-
A Triazole Disulfide Compound Increases the Affinity of Hemoglobin for Oxygen and Reduces the Sickling of Human Sickle Cells.Mol Pharm. 2018 May 7;15(5):1954-1963. doi: 10.1021/acs.molpharmaceut.8b00108. Epub 2018 Apr 18. Mol Pharm. 2018. PMID: 29634905 Free PMC article.
-
GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease.Br J Haematol. 2016 Oct;175(1):141-53. doi: 10.1111/bjh.14214. Epub 2016 Jul 5. Br J Haematol. 2016. PMID: 27378309
-
Therapeutic strategies to alter the oxygen affinity of sickle hemoglobin.Hematol Oncol Clin North Am. 2014 Apr;28(2):217-31. doi: 10.1016/j.hoc.2013.11.001. Epub 2014 Jan 21. Hematol Oncol Clin North Am. 2014. PMID: 24589263 Free PMC article. Review.
-
Interactions of an Anti-Sickling Drug with Hemoglobin in Red Blood Cells from a Patient with Sickle Cell Anemia.Bioconjug Chem. 2019 Mar 20;30(3):568-571. doi: 10.1021/acs.bioconjchem.9b00130. Epub 2019 Feb 28. Bioconjug Chem. 2019. PMID: 30794381 Free PMC article.
-
Targeting HbS Polymerization.Semin Hematol. 2018 Apr;55(2):53-59. doi: 10.1053/j.seminhematol.2018.04.012. Epub 2018 Apr 27. Semin Hematol. 2018. PMID: 30616807 Review.
Cited by
-
A Triazole Disulfide Compound Increases the Affinity of Hemoglobin for Oxygen and Reduces the Sickling of Human Sickle Cells.Mol Pharm. 2018 May 7;15(5):1954-1963. doi: 10.1021/acs.molpharmaceut.8b00108. Epub 2018 Apr 18. Mol Pharm. 2018. PMID: 29634905 Free PMC article.
-
Sickle Cell Anemia and Its Phenotypes.Annu Rev Genomics Hum Genet. 2018 Aug 31;19:113-147. doi: 10.1146/annurev-genom-083117-021320. Epub 2018 Apr 11. Annu Rev Genomics Hum Genet. 2018. PMID: 29641911 Free PMC article. Review.
-
HypoxyStat, a small-molecule form of hypoxia therapy that increases oxygen-hemoglobin affinity.Cell. 2025 Mar 20;188(6):1580-1588.e11. doi: 10.1016/j.cell.2025.01.029. Epub 2025 Feb 17. Cell. 2025. PMID: 39965572 Free PMC article.
-
Modulating hemoglobin allostery for treatment of sickle cell disease: current progress and intellectual property.Expert Opin Ther Pat. 2022 Feb;32(2):115-130. doi: 10.1080/13543776.2022.1994945. Epub 2021 Nov 1. Expert Opin Ther Pat. 2022. PMID: 34657559 Free PMC article. Review.
-
Rapid and reproducible characterization of sickling during automated deoxygenation in sickle cell disease patients.Am J Hematol. 2019 May;94(5):575-584. doi: 10.1002/ajh.25443. Epub 2019 Mar 8. Am J Hematol. 2019. PMID: 30784099 Free PMC article.
References
-
- Abdella PM, Ritchey JM, Tam JW, Klotz IM. Glycosylation of hemoglobin S by reducing sugars and its effect on gelation. Biochim Biophys Acta. 1977;490:462–70. - PubMed
-
- Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128:552–561. - PubMed
-
- Abdulmalik O, Ghatge MS, Musayev FN, Parikh A, Chen Q, Yang J, Nnamani I, Danso-Danquah R, Eseonu DN, Asakura T, Abraham DJ, Venitz J, Safo MK. Crystallographic analysis of human hemoglobin elucidates the structural basis of the potent and dual antisickling activity of pyridyl derivatives of vanillin. Acta Crystallographica. Section D, Biological Crystallography. 2011;67:920–928. - PMC - PubMed
-
- Abdulmalik O, Deshpande T, Ghatge M, Zhang Y, Venitz J, Parikh A, Safo MK. Novel structurally-modified allosteric effectors of hemoglobin exhibit superior antisickling properties. Blood. 2014;124:218.
-
- Abraham DJ, Mehanna AS, Wireko FC, Whitney J, Thomas RP, Orringer EP. Vanillin, a potential agent for the treatment of sickle cell anemia. Blood. 1991;77:1334–1341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

