Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract
- PMID: 27605301
- PMCID: PMC5015057
- DOI: 10.1038/srep32916
Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract
Abstract
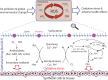
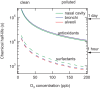
Air pollution can cause oxidative stress and adverse health effects such as asthma and other respiratory diseases, but the underlying chemical processes are not well characterized. Here we present chemical exposure-response relations between ambient concentrations of air pollutants and the production rates and concentrations of reactive oxygen species (ROS) in the epithelial lining fluid (ELF) of the human respiratory tract. In highly polluted environments, fine particulate matter (PM2.5) containing redox-active transition metals, quinones, and secondary organic aerosols can increase ROS concentrations in the ELF to levels characteristic for respiratory diseases. Ambient ozone readily saturates the ELF and can enhance oxidative stress by depleting antioxidants and surfactants. Chemical exposure-response relations provide a quantitative basis for assessing the relative importance of specific air pollutants in different regions of the world, showing that aerosol-induced epithelial ROS levels in polluted megacity air can be several orders of magnitude higher than in pristine rainforest air.
Figures

References
-
- Monks P. S. et al. Atmospheric composition change - global and regional air quality. Atmos. Environ. 43, 5268–5350 (2009).
-
- Pöschl U. & Shiraiwa M. Multiphase Chemistry at the Atmosphere–Biosphere Interface Influencing Climate and Public Health in the Anthropocene. Chem. Rev. 115, 4440–4475 (2015). - PubMed
-
- Pope C. A. & Dockery D. W. Health effects of fine particulate air pollution: lines that connect. J. Air Waste Manag. Assoc. 56, 709–742 (2006). - PubMed
-
- Brunekreef B. & Holgate S. T. Air pollution and health. Lancet 360, 1233–1242 (2002). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

