Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis
- PMID: 27605370
- PMCID: PMC5365077
- DOI: 10.1002/jor.23420
Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis
Abstract
Obesity and associated metabolic diseases collectively referred to as the metabolic syndrome increase the risk of skeletal and synovial joint diseases, including osteoarthritis (OA). The relationship between obesity and musculoskeletal diseases is complex, involving biomechanical, dietary, genetic, inflammatory, and metabolic factors. Recent findings illustrate how changes in cellular metabolism and metabolic signaling pathways alter skeletal development, remodeling, and homeostasis, especially in response to biomechanical and inflammatory stressors. Consequently, a better understanding of the energy metabolism of diarthrodial joint cells and tissues, including bone, cartilage, and synovium, may lead to new strategies to treat or prevent synovial joint diseases such as OA. This rationale was the basis of a workshop presented at the 2016 Annual ORS Meeting in Orlando, FL on the emerging role of metabolic signaling in synovial joint remodeling and OA. The topics we covered included (i) the relationship between metabolic syndrome and OA in clinical and pre-clinical studies; (ii) the effect of biomechanical loading on chondrocyte metabolism; (iii) the effect of Wnt signaling on osteoblast carbohydrate and amino acid metabolism with respect to bone anabolism; and (iv) the role of AMP-activated protein kinase in chondrocyte energetic and biomechanical stress responses in the context of cartilage injury, aging, and OA. Although challenges exist for measuring in vivo changes in synovial joint tissue metabolism, the findings presented herein provide multiple lines of evidence to support a central role for disrupted cellular energy metabolism in the pathogenesis of OA. © 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 34:2048-2058, 2016.
Keywords: bone; cartilage; metabolic syndrome; obesity; osteoarthritis.
© 2016 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Conflict of interest statement
RKJ has financial interests in Beartooth Biotech that has licensed a stoichiometric model of energy metabolism. FL, RLB, and TMG have no conflicts to disclose.
Figures
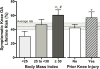

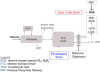
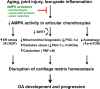
References
-
- Brooks PM. The burden of musculoskeletal disease–a global perspective. Clin Rheumatol. 2006;25(6):778–781. - PubMed
-
- Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, Middeldorp S, Huizinga TW, Kloppenburg M. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761–765. - PubMed
-
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthritis Cartilage. 2013;21(1):16–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

